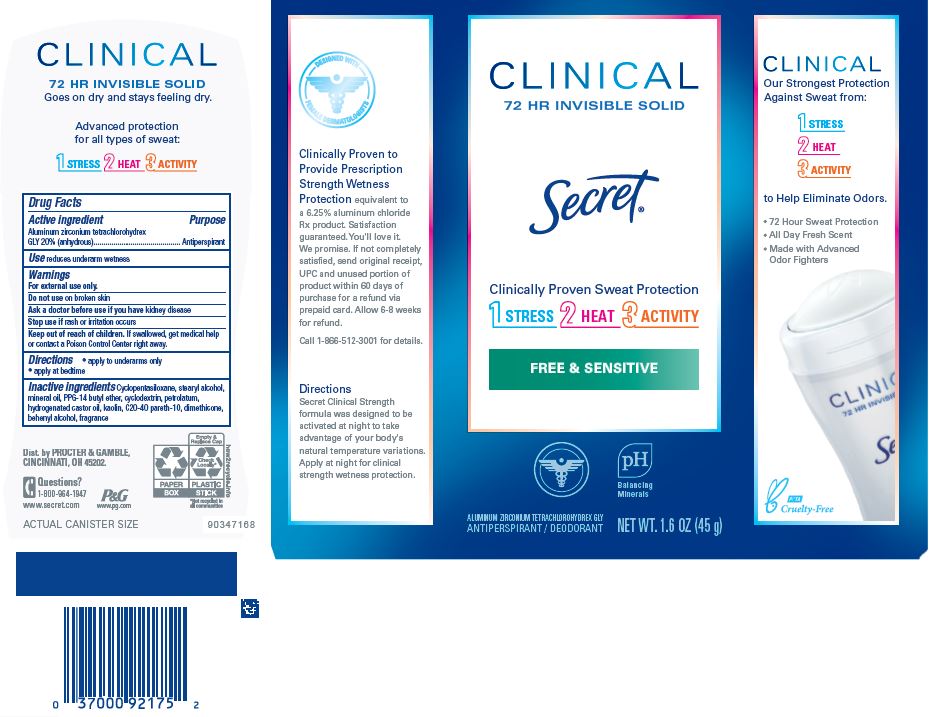
Secret ® Clinical Strength Invisible Solid Free and Sensitive
Secret Clinical Strength Fresh and Sensitive Invisible by
Drug Labeling and Warnings
Secret Clinical Strength Fresh and Sensitive Invisible by is a Otc medication manufactured, distributed, or labeled by The Procter & Gamble Manufacturing Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SECRET CLINICAL STRENGTH FRESH AND SENSITIVE INVISIBLE- aluminum zirconium tetrachlorohydrex gly stick
The Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Secret
® Clinical Strength Invisible Solid
Free and Sensitive
| SECRET CLINICAL STRENGTH FRESH AND SENSITIVE INVISIBLE
aluminum zirconium tetrachlorohydrex gly stick |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |
Revised: 6/2023
Document Id: fdf318a8-cb6c-6bb9-e053-6394a90a56dd
Set id: b5fbf0c5-e159-4fb8-e053-2a95a90ad337
Version: 3
Effective Time: 20230612
The Pr
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.