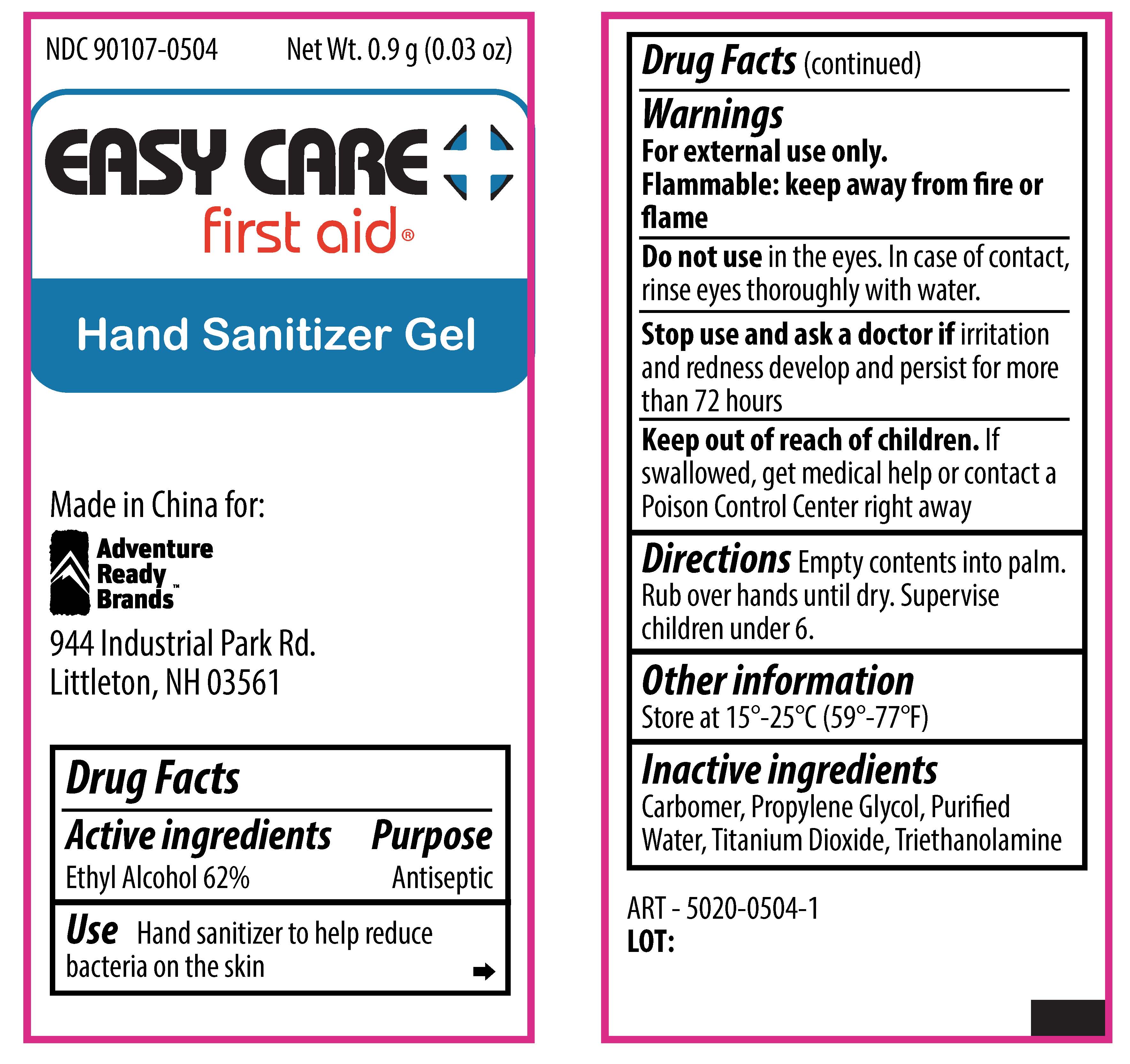
Easy Care First Aid Hand Sanitizer
Easy Care First Aid Hand Sanitizer by
Drug Labeling and Warnings
Easy Care First Aid Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Adventure Ready Brands, Tender Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
EASY CARE FIRST AID HAND SANITIZER- alcohol gel
Adventure Ready Brands
----------
Easy Care First Aid Hand Sanitizer
| EASY CARE FIRST AID HAND SANITIZER
alcohol gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Adventure Ready Brands (064437304) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Tender Corporation | 064437304 | manufacture(90107-0504) | |
Revised: 12/2024
Document Id: 28f1e9f3-d53a-82df-e063-6294a90a9de5
Set id: b620a98a-dd0b-2d8e-e053-2995a90a12fd
Version: 3
Effective Time: 20241210
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.