Neuraxcin Neuromethacin, Pain Relief Roll-on
Neuraxcin Neuromethacin by
Drug Labeling and Warnings
Neuraxcin Neuromethacin by is a Otc medication manufactured, distributed, or labeled by Sola Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NEURAXCIN NEUROMETHACIN- menthol roll-on liquid
Sola Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
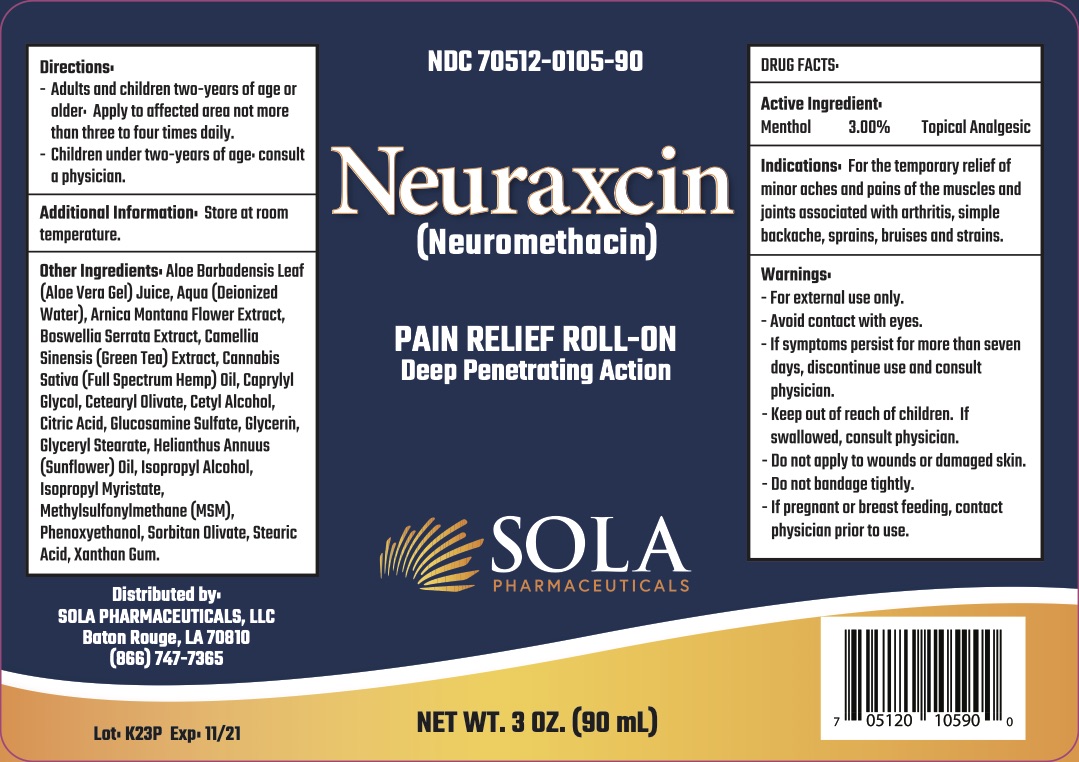
Neuraxcin Neuromethacin, Pain Relief Roll-on
Indications
For the temporary relief of minor aches and pains of the muscles and joints associated with arthritis, simple backache, sprains, bruises and strains.
Warnings
-For external use only.
-Avoid contact with eyes.
-If symptoms persist for more than seven days, discontinue use and consult physician.
Keep out of reach of children
-If swallowed, consult physician.
-Do not apply to wounds or damaged skin.
-Do not bandage tightly.
Directions
-Adults and children two-years of age or older. Apply to affected area not more than three to four times daily.
-Children under two-years of age, consult a physician.
Other Ingredients
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Arnica Montant Flower Extract, Boswellia Serrata Extract, Camellia Sinensis (Green Tea) Extract, Cannabis Sativa (Full Spectrum Hemp) Oil, Caprylyl Glycol, Cetearyl Olivate, Cetyl Alcohol, Citric Acid, Glucosamine Sulfate, Glycerin, Glyceryl Stearate, Helianthus Annus (Sunflower) Oil, Isopropyl Alcohol, Isopropyl Myristate, Methylsufonylmethane (MSM), Phenoxyethanol, Sorbitan Olivate, Stearic Acid, Xanthan Gum.
| NEURAXCIN NEUROMETHACIN
menthol roll-on liquid |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Sola Pharmaceuticals (080121345) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.