Ephedrine Sulfate Capsules, USP
Ephedrine Sulfate by
Drug Labeling and Warnings
Ephedrine Sulfate by is a Otc medication manufactured, distributed, or labeled by West-ward Pharmaceutical Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
EPHEDRINE SULFATE- ephedrine sulfate capsule
Hikma Pharmaceuticals USA Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Ephedrine Sulfate Capsules, USP
Boxed Warning
FOR YOUR PROTECTION, DO NOT USE IF SEAL OVER MOUTH OF BOTTLE IS BROKEN OR MISSING. CAPUSLES ARE SEALED WITH A RED GELATIN BAND
Indications
For temporary relief of shortness of breath, tightness of chest, and wheezing due to bronchial asthma. For the temporary relief of bronchial asthma. Eases breathing for asthma patients by reducing spasms of bronchial muscles.
Warnings
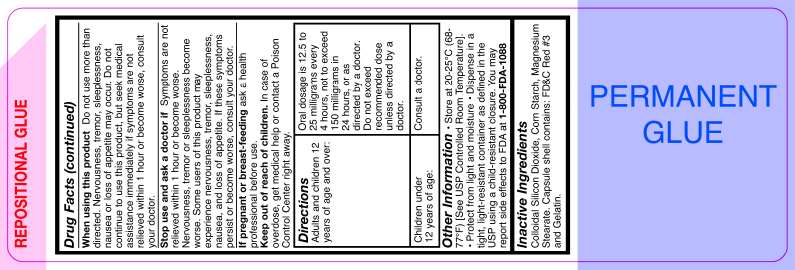
Do not use this product unless a diagnosis of asthma has been made by a doctor. Do not use this product if you have heart disease, high blood pressure, thyroid disease, diabetes, or difficulty in urination due to enlargement of the prostate gland unless directed by a doctor. Do not use this product if you have ever been hospitalized for asthma or if you are taking and prescription drug for asthma or if you are taking and prescription drug for asthma unless directed by a doctor.
Drug Interaction precaution
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor of pharmacist before taking this product.
Ask a doctor before use if you have
heart disease
high blood pressure
thyroid disease
diabetes
trouble urinating due to an enlarged prostate gland
When using this product
Do not use more than directed. Nervousness, tremor, sleeplessness, nausea or loss of appetite may occur. Do not continue to use this product, but seek medical assistance immediately if symptoms are not relieved within 1 hour or become worse, consult your doctor.
Stop use and ask a doctor if
Symptoms are not relieved within 1 hour or become worse. Nervousness, tremor or sleeplessness become worse. Some users of this product may experience nervousness, tremor, sleeplessness, nausea, and loss of appetite. If these symptoms persist or become worse, consult your doctor.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
|
Adults and children 12 years of age and over: |
Oral dosage is 12.5 to 25 milligrams every 4 hours, not to exceed 150 milligrams in 24 hours, or as directed by a doctor. Do not exceed recommended dose unless directed by a doctor. |
|||
| Children under 12 years of age: | Consult a doctor. |
Other information
Store at 20-25°C (68-77°F) [See USP Controlled Room Temperature]. Protect from light and moisture. Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure. You may report side effects to FDA at 1-800-FDA-1088.
| EPHEDRINE SULFATE
ephedrine sulfate capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Hikma Pharmaceuticals USA Inc. (001230762) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hikma Pharmaceuticals USA Inc. | 001230762 | MANUFACTURE(0143-3145) | |