Triamazole by PureTek Corporation / Teligent Pharma, Inc. Triamazole
Triamazole by
Drug Labeling and Warnings
Triamazole by is a Prescription medication manufactured, distributed, or labeled by PureTek Corporation, Teligent Pharma, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TRIAMAZOLE- econazole nitrate ,triamcinolone acetonide
PureTek Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Triamazole
DESCRIPTION
Econazole Nitrate Cream contains the antifungal agent, econazole nitrate 1% in a water miscible base consisting of pegoxol 7 stearate, peglicol 5 oleate, mineral oil, benzoic acid, butylated hydroxyanisole, and purified water. The white to off-white soft cream is for topical use only.
Chemically, econazole nitrate is 1-[2-{(4-chloro-phenyl) methoxy}-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole mononitrate. Its structure is as follows:
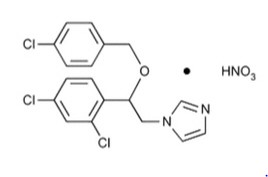
CLINICAL PHARMACOLOGY
After topical application to the skin of normal subjects, systemic absorption of econazole nitrate is extremely low. Although most of the applied drug remains on the skin surface, drug concentrations were found in the stratum corneum which, by far, exceeded the minimum inhibitory concentration for dermatophytes. Inhibitory concentrations were achieved in the epidermis and as deep as the middle region of the dermis. Less than 1% of the applied dose was recovered in the urine and feces.
Microbiology
Econazole nitrate has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
| Dermatophytes | Yeasts |
| Epidermophyton floccosum | Candida albicans |
| Microsporum audouini | Malassezia furfur |
| Microsporum canis | |
| Microsporum gypseum | |
| Trichophyton mentagrophytes | |
| Trichophyton rubrum | |
| Trichophyton tonsurans |
Econazole nitrate exhibits broad-spectrum antifungal activity against the following organisms in vitro, but the clinical significance of these data is unknown.
|
Dermatophytes | Yeasts |
| Trichophyton verrucosum | Candida guillermondii |
| Candida parapsilosis | |
| Candida tropicalis |
INDICATIONS AND USAGE
Econazole Nitrate Cream is indicated for topical application in the treatment of tinea pedis, tinea cruris, and tinea corporis caused by Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton tonsurans, Microsporum canis, Microsporum audouini, Microsporum gypseum, and Epidermophyton floccosum, in the treatment of cutaneous candidiasis, and in the treatment of tinea versicolor
CONTRAINDICATIONS
Econazole Nitrate Cream is contraindicated in individuals who have shown hypersensitivity to any of its ingredients.
Carcinogenicity Studies
Long-term animal studies to determine carcinogenic potential have not been performed.
Oral administration of econazole nitrate in rats has been reported to produce prolonged gestation. Intravaginal administration in humans has not shown prolonged gestation or other adverse reproductive effects attributable to econazole nitrate therapy.
Fertility (Reproduction)
Oral administration of econazole nitrate in rats has been reported to produce prolonged gestation. Intravaginal administration in humans has not shown prolonged gestation or other adverse reproductive effects attributable to econazole nitrate therapy.
Pregnancy
Econazole nitrate has not been shown to be teratogenic when administered orally to mice, rabbits or rats. Fetotoxic or embryotoxic effects were observed in Segment I oral studies with rats receiving 10 to 40 time the human dermal dose. Similar effects were observed in Segment II or Segment III studies with mice, rabbits and/or rats receiving oral doses 80 or 40 time the human dermal dose.
Econazole nitrate should be used in the first trimester of pregnancy only when the physician considers it essential to the welfare of the patient. The drug should be used during the second and third trimesters of pregnancy only if clearly needed.
Nursing Mothers
It is not known whether econazole nitrate is excreted in human milk. Following oral administration of econazole nitrate to lactating rats, econazole and/or metabolites were excreted in milk and were found in nursing pups. Also, in lactating rats receiving large oral doses (40 or 80 times the human dermal dose), there was a reduction in post partum viability of pups and survival to weaning; however, at these high doses, maternal toxicity was present and may have been a contributing factor. Caution should be exercised when econazole nitrate is administered to a nursing woman.
ADVERSE REACTIONS
During clinical trials, approximately 3% of patients treated with econazole nitrate 1% cream reported side effects thought possibly to be due to the drug, consisting mainly of burning, itching, stinging, and erythema. One case of pruritic rash has also been reported.
To report suspected adverse reactions, contact Teligent Pharma, Inc. at 1-856-697-1441, or FDA at 1-800-FDA-1088 or 1-800-332-1088 or www.fda.gov/medwatch.
OVERDOSE
Overdosage of econazole nitrate in humans has not been reported to date. In mice, rats, guinea pigs and dogs, the oral LD 50 values were found to be 462, 668, 272, and >160 mg/kg, respectively.
DOSAGE AND ADMINISTRATION
Sufficient Econazole Nitrate Cream 1% should be applied to cover affected areas once daily in patients with tinea pedis, tinea cruris, tinea corporis, and tinea versicolor, and twice daily (morning and evening) in patients with cutaneous candidiasis.
Early relief of symptoms is experienced by the majority of patients and clinical improvement may be seen fairly soon after treatment is begun; however, candidal infections and tinea cruris and corporis should be treated for two weeks and tinea pedis for one month in order to reduce the possibility of recurrence. If a patient shows no clinical improvement after the treatment period, the diagnosis should be redetermined. Patients with tinea versicolor usually exhibit clinical and mycological clearing after two weeks of treatment.
HOW SUPPLIED
Econazole Nitrate Cream 1% is supplied in the following:
15 gram tubes (NDC: 52565-022-15)
30 gram tubes (NDC: 52565-022-30)
85 gram tubes (NDC: 52565-022-85)
DESCRIPTION
The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents. The steroids in this class include triamcinolone acetonide. Triamcinolone acetonide is designated chemically as 9-Fluoro-11β, 16α, 17,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone. C 24H 31FO 6. M.W. 434.51; CAS Reg. No. 76-25-5.
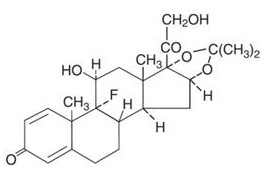
Each gram of Triamcinolone Acetonide Ointment USP, 0.1% contains 1mg triamcinolone acetonide in an ointment base of light mineral oil and white petrolatum.
CLINICAL PHARMACOLOGY
Topical corticosteroids share anti-inflammatory, antipruritic, and vasoconstrictive actions. The mechanism of the anti- inflammatory activity of the topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Pharmacokinetics -
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids. Thus, occlusive dressings may be a valuable therapeutic adjunct for the treatment of resistant dermatoses (see DOSAGE AND ADMINISTRATION).
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids.
Corticosteroids are bound to plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
INDICATIONS AND USAGE
Triamcinolone Acetonide Ointment is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
CONTRAINDICATIONS
Topical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparations.
PRECAUTIONS
General
Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing’s syndrome, hyperglycemia, and glucosuria in some patients.
Conditions that augment systemic absorption include the application of the more potent steroids, use over large surface areas, prolonged use, and the addition of occlusive dressings.
Therefore, patients receiving a large dose of any potent topical steroid applied to a large surface area or under an occlusive dressing should be evaluated periodically for evidence of HPA axis suppression by using the urinary free cortisol and ACTH stimulation tests, and for impairment of thermal homeostasis. If HPA axis suppression or elevation of the body temperature occurs, an attempt should be made to withdraw the drug, to reduce the frequency of application, substitute a less potent steroid, or use a sequential approach when utilizing the occlusive technique. Recovery of HPA axis function and thermal homeostasis are generally prompt and complete upon discontinuation of the drug. Infrequently, signs and symptoms of steroid withdrawal may occur, requiring supplemental systemic corticosteroids. Occasionally, a patient may develop a sensitivity reaction to a particular occlusive dressing material or adhesive and a substitute material may be necessary.
Children may absorb proportionally larger amounts of topical corticosteroids and thus be more susceptible to systemic toxicity (see PRECAUTIONS, Pediatric Use). If irritation develops, topical corticosteroids should be discontinued and appropriate therapy instituted.
In the presence of dermatological infections, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly, the corticosteroid should be discontinued until the infection has been adequately controlled.
These preparations are not for ophthalmic use.
Information for Patients
Patients using topical corticosteroids should receive the following information and instructions:
1. This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
2. Patients should be advised not to use this medication for any disorder other than for which it was prescribed.
3. The treated skin area should not be bandaged or otherwise covered or wrapped as to be occlusive unless directed by the physician.
4. Patients should report any signs of local adverse reactions, especially under occlusive dressing.
5. Parents of pediatric patients should be advised not to use tight-fitting diapers or plastic pants on a child being treated in the diaper area, as these garments may constitute occlusive dressings.
Laboratory Tests
A urinary free cortisol test and ACTH stimulation test may be helpful in evaluating HPA axis suppression.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of topical corticosteroids.
Studies to determine mutagenicity with prednisolone and hydrocortisone showed negative results.
Pregnancy:
Pregnancy Category C -
Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals. There are no adequate and well-controlled studies in pregnant women on teratogenic effects from topically applied corticosteroids. Therefore, topical corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Drugs of this class should not be used extensively on pregnant patients, in large amounts, or for prolonged periods of time.
Nursing Mothers
It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered corticosteroids are secreted into breast milk in quantities not likely to have a deleterious effect on the infant. Nevertheless, caution should be exercised when topical corticosteroids are administered to a nursing woman.
Pediatric Use
Pediatric patients may demonstrate greater susceptibility to topical corticosteroid-induced HPA axis suppression and Cushing’s syndrome than mature patients because of a larger skin surface area to body weight ratio.
HPA axis suppression, Cushing’s syndrome, and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include linear growth retardation, delayed weight gain, low plasma cortisol levels, and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Administration of topical corticosteroids to children should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of children.
ADVERSE REACTIONS
The following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings (reactions are listed in approximate decreasing order of occurrence): burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae, and miliaria.
To report SUSPECTED ADVERSE REACTIONS, contact Teligent Pharma, Inc. at 1-856-697-1441, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
Topically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects (see PRECAUTIONS, General).
DOSAGE AND ADMINISTRATION
Apply a thin film to the affected area two to three times daily.
Occlusive Dressing Technique
Occlusive dressings may be used for the management of psoriasis or other recalcitrant conditions. Apply a thin film of ointment to the lesion, cover with a pliable nonporous film, and seal the edges. If needed, additional moisture may be provided by covering the lesion with a dampened clean cotton cloth before the nonporous film is applied or by briefly wetting the affected area with water immediately prior to applying the medication.
The frequency of changing dressings is best determined on an individual basis. It may be convenient to apply Triamcinolone Acetonide Ointment under an occlusive dressing in the evening and to remove the dressing in the morning (i.e., 12-hour occlusion). When utilizing the 12-hour occlusion regimen, the additional ointment should be applied, without occlusion, during the day. Reapplication is essential at each dressing change.
If an infection develops, the use of occlusive dressings should be discontinued and appropriate antimicrobial therapy instituted.
HOW SUPPLIED
Triamcinolone Acetonide Ointment USP, 0.1% is supplied in the following sizes:
15 g tube – NDC: 52565-014-15
80 g tube – NDC: 52565-014-80
1 lb (454 g) jar – NDC: 52565-014-26
| TRIAMAZOLE
econazole nitrate ,triamcinolone acetonide kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - PureTek Corporation (785961046) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Teligent Pharma, Inc. | 011036910 | manufacture(52565-022, 52565-014) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PureTek Corporation | 785961046 | pack(59088-777) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
