LiProtect SPF 35 (69219-109) - DELIST
LiProtect SPF 35 by
Drug Labeling and Warnings
LiProtect SPF 35 by is a Otc medication manufactured, distributed, or labeled by Science of Skincare, Micro Connection Enterprises INC, West Coast Cosmetics. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
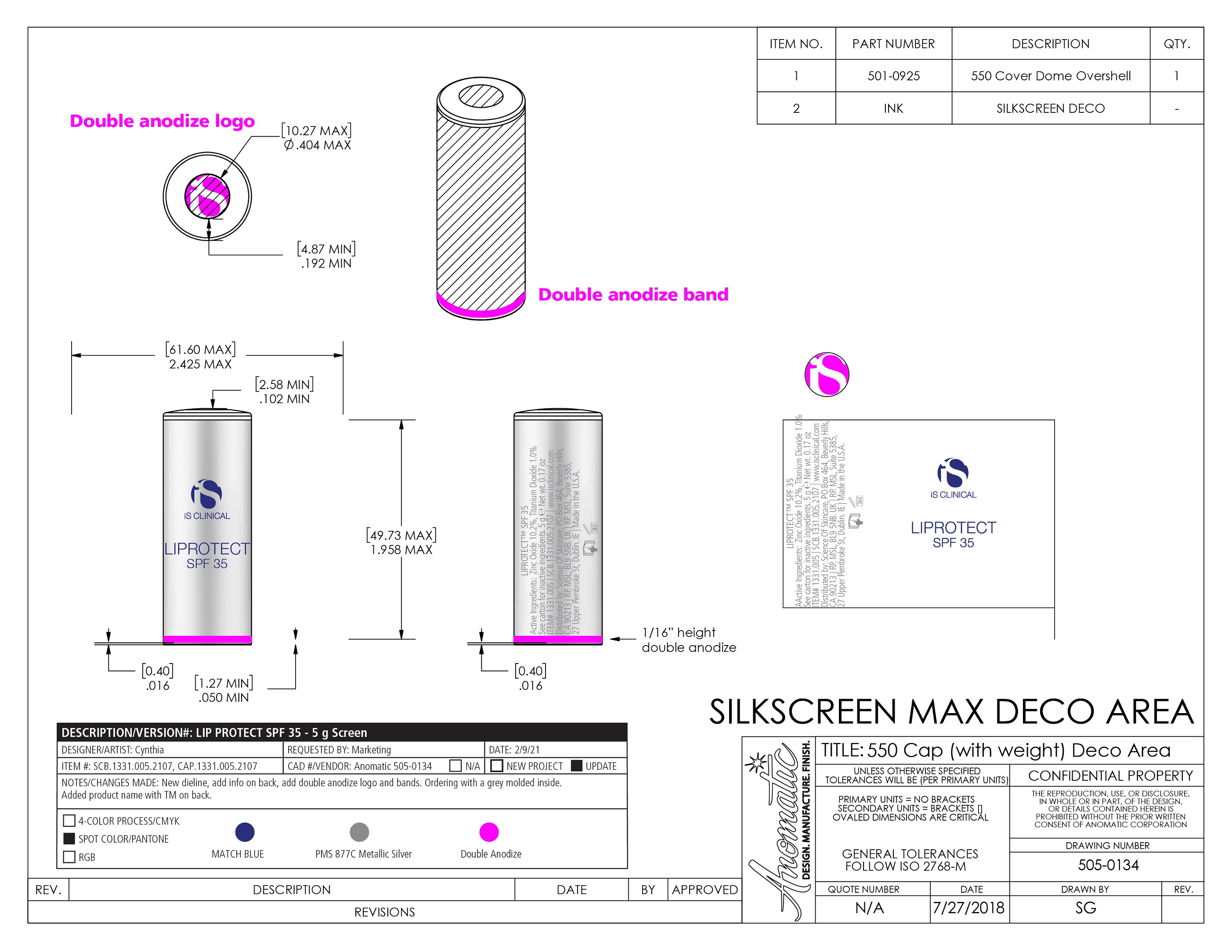
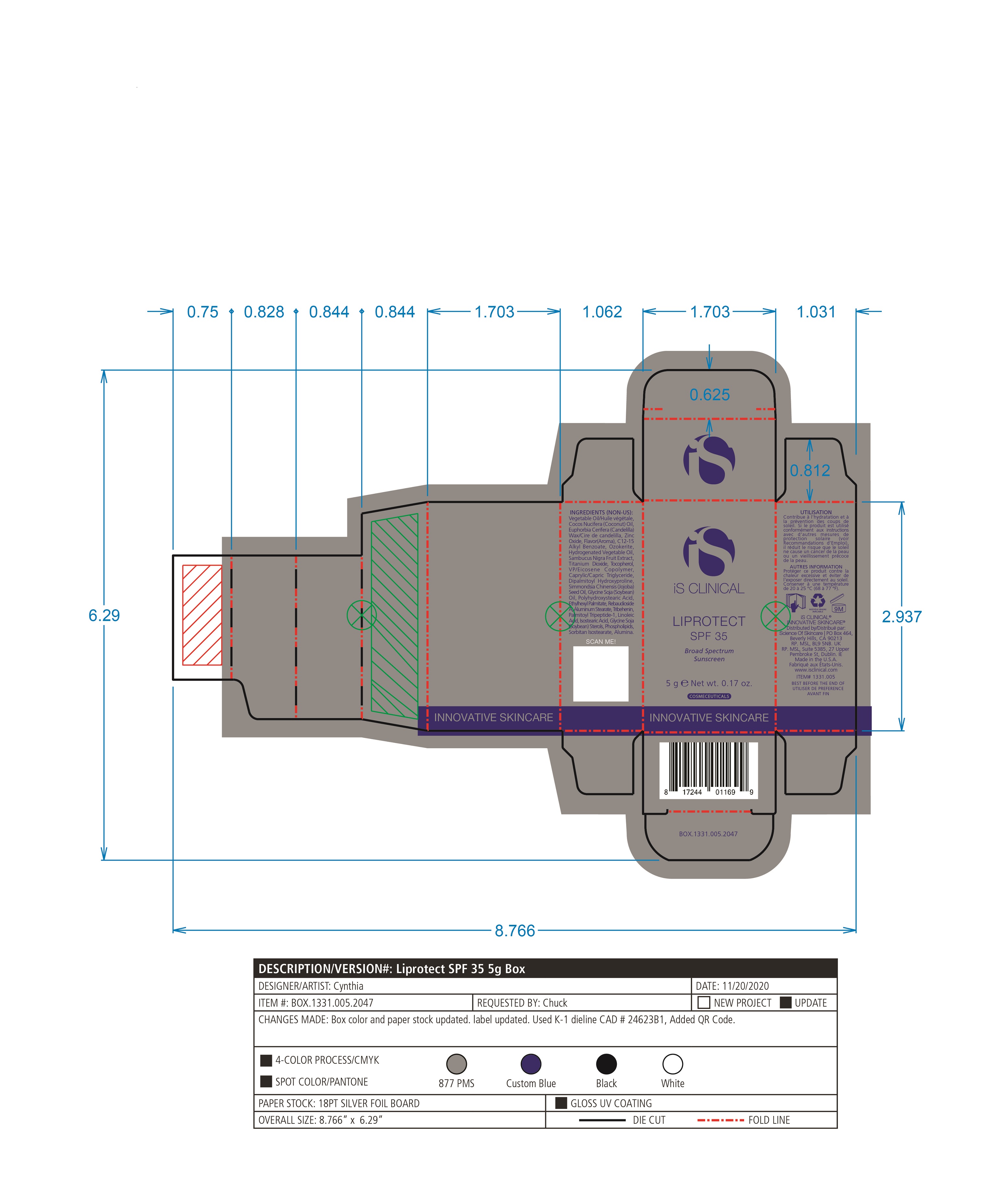
LIPROTECT SPF 35- zinc oxide, titanium dioxide stick
Science of Skincare
----------
LiProtect SPF 35 (69219-109) - DELIST
Helps prevent sunburn
If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Apply liberally 15 minutes before sun exposure
Use a water resistance sunscreen if swimming or sweating.
Reapply at least every 2 hours
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a board spectrum SPF of 15 or higher and other sun protection measures including: limit time in sun, especially from 10am to 2pm. wear long-sleeve shirts, pants, hats, and sunglasses. Children under the age of 6 months. Ask a doctor.
Vegetable Oil, Cocos Nucifera (Coconut) Oil, Euphorbia Cerifera (Candelilla) Wax, Flavor(Aroma), C12-15 Alkyl Benzoate, Ozokerite, Hydrogenated Vegetable Oil, Sambucus Nigra Fruit Extract, Tocopherol, VP/Eicosene Copolymer, Caprylic/Capric Triglyceride, Dipalmitoyl Hydroxyproline, Simmondsia Chinensis (Jojoba) Seed Oil, Glycine Soja (Soybean) Oil, Polyhydroxystearic Acid, Ethylhexyl Palmitate, Rebaudioside A, Aluminum Stearate, Tribehenin, Palmitoyl Tripeptide-1, Linoleic Acid, Isostearic Acid, Glycine Soja (Soybean) Sterols, Phospholipids, Sorbitan Isostearate, Alumina.
| LIPROTECT SPF 35
zinc oxide, titanium dioxide stick |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Science of Skincare (006251958) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.