ARTIFICIAL TEARS- polyvinyl alcohol solution/ drops
Artificial Tears by
Drug Labeling and Warnings
Artificial Tears by is a Otc medication manufactured, distributed, or labeled by MWI, Akorn, Inc., Akorn, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
- Do not use if imprinted seal on the bottle neck is broken or missing.
- Do not use if solution changes color or becomes cloudy.
- To avoid contamination, do not touch tip of container to any surface.
- Replace cap after using.
- Directions
- Other information
- Inactive ingredients
- Questions?
-
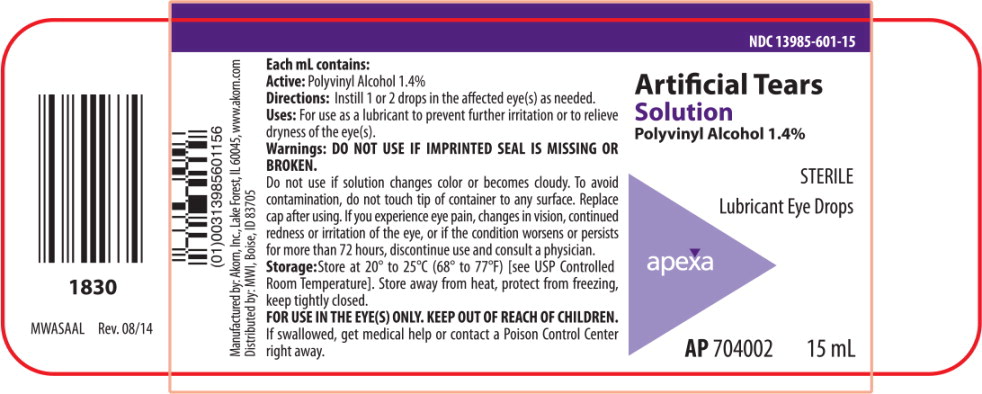
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 13985-601-15
Artificial Tears
Solution
Polyvinyl Alcohol 1.4%
STERILE
Lubricant Eye Drops
Apexa logo
AP 704002 15 mL
-
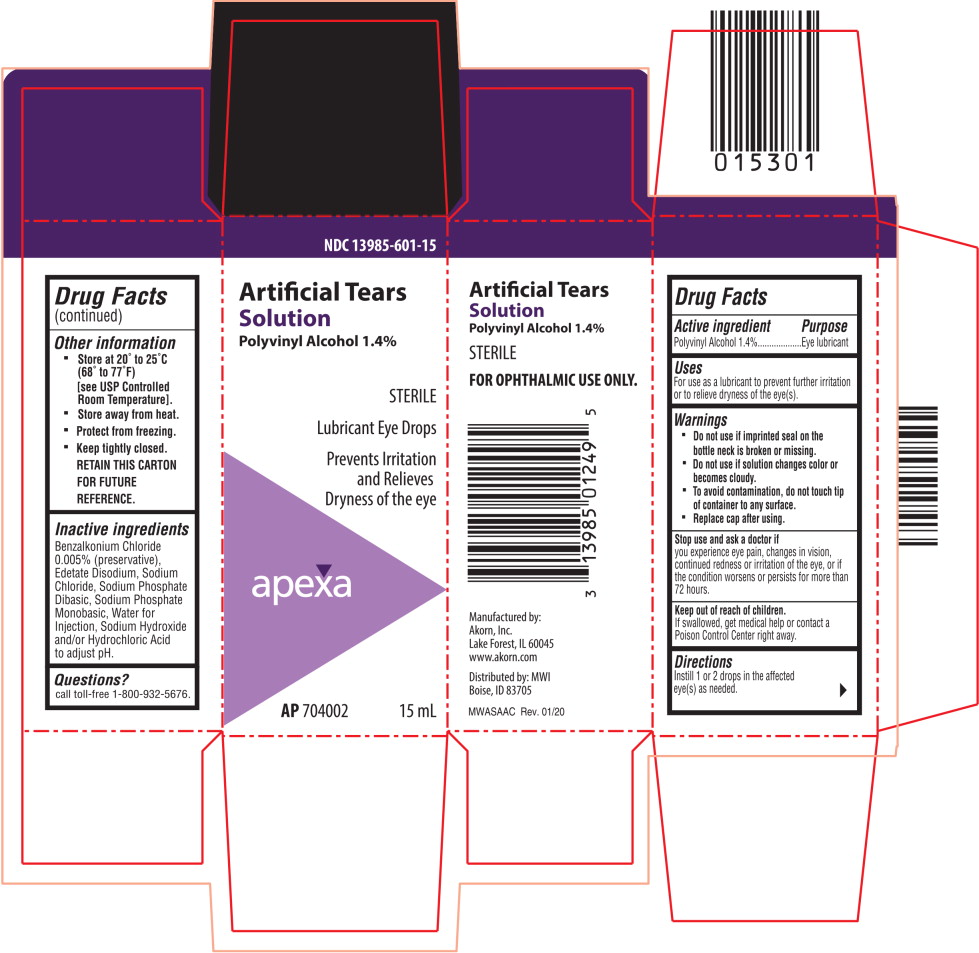
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 13985-601-15
Artificial Tears
Solution
Polyvinyl Alcohol 1.4%
STERILE
Lubricant Eye Drops
Prevents Irritation
and Relieves
Dryness of the eye
Apexa logo
AP 704002 15 mL
-
INGREDIENTS AND APPEARANCE
ARTIFICIAL TEARS
polyvinyl alcohol solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13985-601 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) (POLYVINYL ALCOHOL, UNSPECIFIED - UNII:532B59J990) POLYVINYL ALCOHOL, UNSPECIFIED 14 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) Edetate Disodium (UNII: 7FLD91C86K) Sodium Chloride (UNII: 451W47IQ8X) Sodium Phosphate, Dibasic, Anhydrous (UNII: 22ADO53M6F) Sodium Phosphate, Monobasic, Anhydrous (UNII: KH7I04HPUU) Water (UNII: 059QF0KO0R) Sodium Hydroxide (UNII: 55X04QC32I) Hydrochloric Acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13985-601-15 1 in 1 CARTON 03/23/2015 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 03/23/2015 Labeler - MWI (019926120) Registrant - Akorn, Inc. (062649876) Establishment Name Address ID/FEI Business Operations Akorn, Inc 603980319 MANUFACTURE(13985-601) , ANALYSIS(13985-601) , STERILIZE(13985-601) , PACK(13985-601) , LABEL(13985-601)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.