Colic Calm by Denison Pharmaceuticals, Inc. Colic Calm
Colic Calm by
Drug Labeling and Warnings
Colic Calm by is a Homeopathic medication manufactured, distributed, or labeled by Denison Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
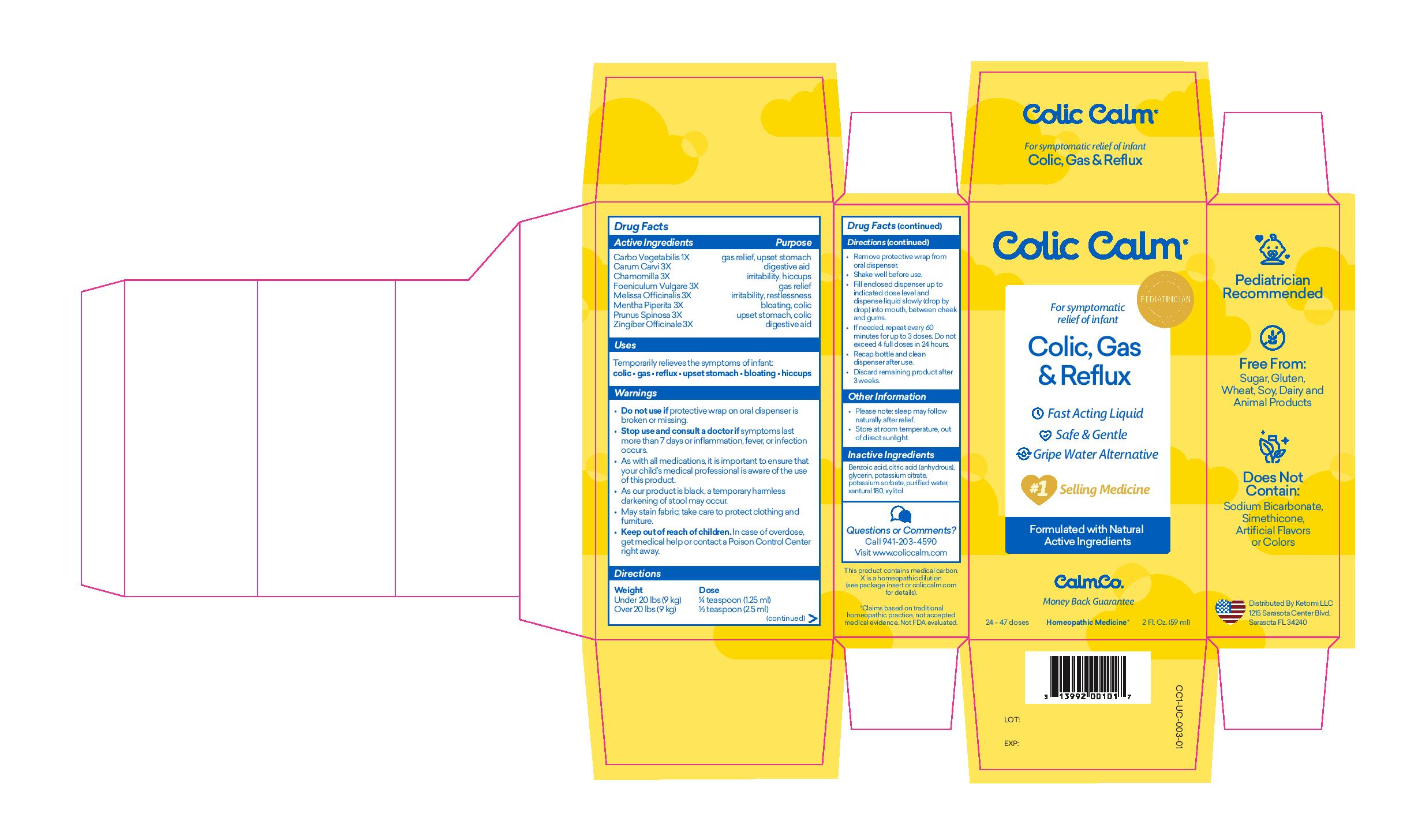
COLIC CALM- carbo vegetababilis, carum carvi, chamomilla, foeniculum vulgare, melissa officinalis, mentha piperita, prunus spinosa, zingiber officinale liquid
Denison Pharmaceuticals, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Colic Calm
Active Ingredient
Active Ingredients
Carbo Vegetabilis 1x, Carum Carvi 3X, Chamomilla 3X, Foeniculum Vulgare 3X, Melissa Officinalis 3X, Mentha Piperita 3x, Prunus Spinosa 3X, Zingiber Officinale 3X
Purpose
- gas relief, upset stomach
- digestive aid
- irritability, hiccups
- gas relief
- irritability, restlessness
- bloating, colic
- upset stomach, colic
- digestive aid
Uses
Temporarily relieves the symptoms of infant:
colic, gas, reflux, upset stomach, bloating, hiccups
Warnings
- Do not use if protective wrap on oral dispenser is broken or missing.
- Stop use and consult a doctor if symptoms last more than 7 days or inflammation, fever, or infection occurs.
- As with all medications, it is important to ensure that your child’s medical professional is aware of the use of this product.
- As our product is black, a temporary harmless darkening of stool may occur.
- May stain fabric; take care to protect clothing and furniture.
Directions
Weight under 20 lbs (9kg) Dose 1/4 teaspoon (1.25 ml)
Weight over 20 lbs (9kg) Dose 1/2 teaspoon (2.5 ml)
- Remove protective wrap from oral dispenser.
- Shake well before use.
- Fill enclosed dispenser up to indicated dose level and dispense liquid slowly (drop by drop) into mouth, between cheek and gums.
- If needed, repeat every 60 minutes for up to 3 doses. Do not exceed 4 full doses in 24 hours.
- Recap bottle and clean dispenser after use.
- Discard remaining product after 3 weeks.
Other Information
- Please notes: sleep may follow naturally after relief
- Store at room temperature, out of direct sunlight
| COLIC CALM
carbo vegetababilis, carum carvi, chamomilla, foeniculum vulgare, melissa officinalis, mentha piperita, prunus spinosa, zingiber officinale liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Denison Pharmaceuticals, Inc. (001207208) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Denison Pharmaceuticals, Inc. | 001207208 | manufacture(0295-9028) | |
Trademark Results [Colic Calm]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COLIC CALM 98274953 not registered Live/Pending |
CalmCo LLC 2023-11-17 |
 COLIC CALM 90259595 not registered Live/Pending |
CalmCo LLC 2020-10-16 |
 COLIC CALM 88002853 not registered Dead/Abandoned |
KETOMI LLC 2018-06-15 |
 COLIC CALM 87774984 5565044 Live/Registered |
KETOMI LLC 2018-01-29 |
 COLIC CALM 85119554 3965195 Live/Registered |
KETOMI LLC 2010-08-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.