DIBUCAINE by NUGERI LLC / UNIPack DIBUCAINE 1% Ointment
DIBUCAINE by
Drug Labeling and Warnings
DIBUCAINE by is a Otc medication manufactured, distributed, or labeled by NUGERI LLC, UNIPack. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DIBUCAINE- dibucaine ointmentÂ
NUGERI LLC
----------
DIBUCAINE 1% Ointment
Product Image
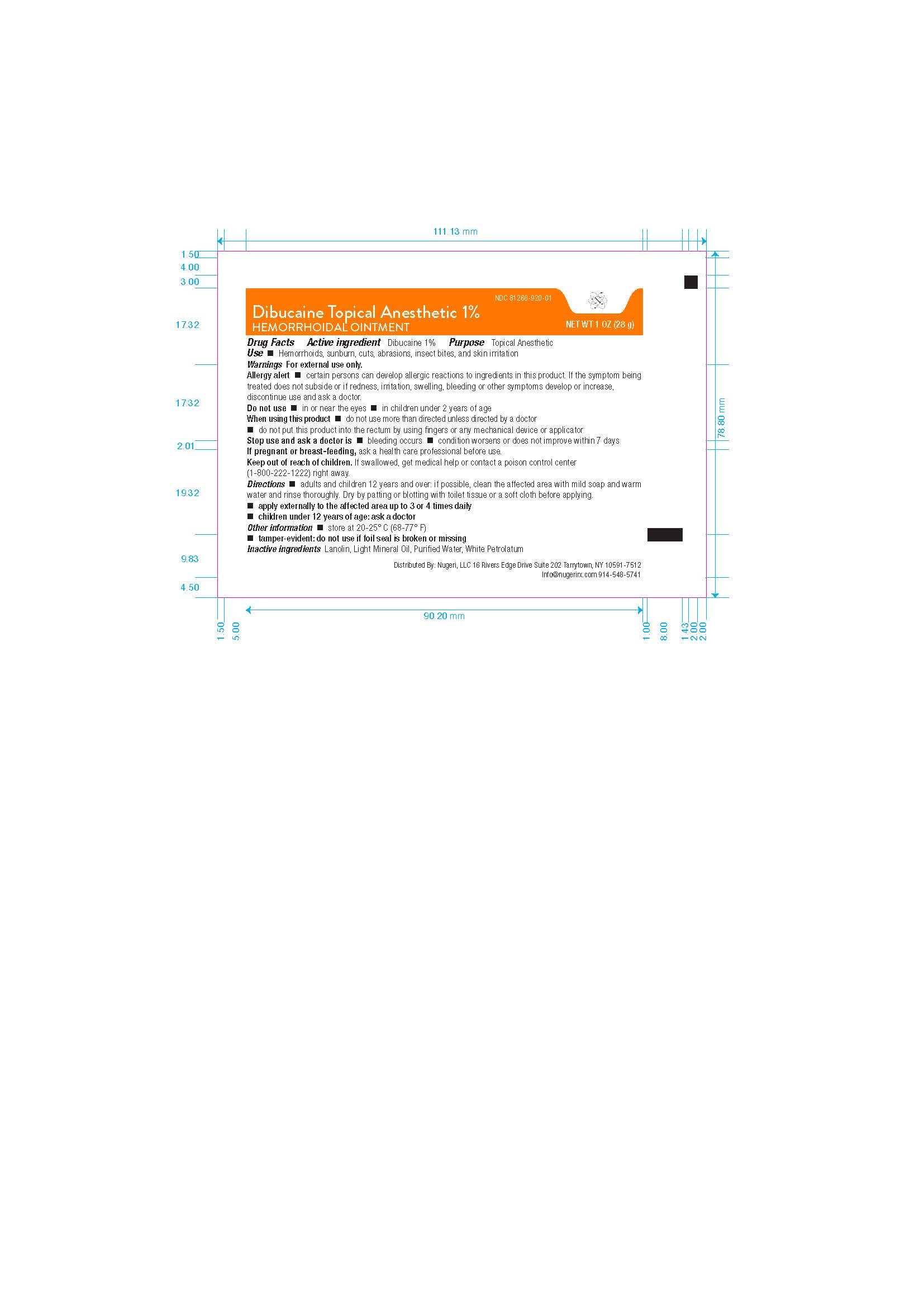
Allery Alert
Certain persons can develop allergic reactions to ingredients in this product. If the symptom being treated does not subside or if redness, irritation, swelling, bleeding, orother symptoms develp or increase, discontinue use and ask a doctor.
When Using This Product
- Do not use more than directed unless directed by a doctor
- Do not put this product into the rectum by using fingers or any mechanical device or applicator
Keep Out Of Reach Of Children
If swallowed, get medical help or contact a poison control center (1-800-222-1222) right away.
Directons
- Adults and children 12 years and older; if possible clean the affected area with mild soap and warm water and rinse thoroughly. Dry by patting or blotting with toliet tissue or soft cloth before applying.
- Apply externally to the affected area up to 3 to 4 times daily
- Children under 12 years of age ask a doctor
Other Information
- Store at 20-25 0 C (68-77 0 F)
- Tamper-evident: do not use if foil seal is broken or missing
| DIBUCAINEÂ
dibucaine ointment |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler -Â NUGERI LLC (117724142) |
| Registrant -Â UNIPack (009248480) |
Revised: 10/2023
Â
Document Id: 0874bae1-eb7b-3c08-e063-6294a90aaba0
Set id: b6ca4519-6c8e-49e0-e053-2995a90af800
Version: 3
Effective Time: 20231024
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.