HEMANGEOL- propranolol hydrochloride solution
HEMANGEOL by
Drug Labeling and Warnings
HEMANGEOL by is a Prescription medication manufactured, distributed, or labeled by Pierre Fabre Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HEMANGEOL safely and effectively. See full prescribing information for HEMANGEOL.
HEMANGEOL® (propranolol hydrochloride oral solution)
Initial U.S. Approval: 1967INDICATIONS AND USAGE
HEMANGEOL oral solution is a beta-adrenergic blocker indicated for the treatment of proliferating infantile hemangioma requiring systemic therapy. ( 1)
DOSAGE AND ADMINISTRATION
- Initiate treatment at ages 5 weeks to 5 months. ( 2)
- Starting dose is 0.15 mL/kg (0.6 mg/kg) twice daily. After 1 week, increase dose to 0.3 mL/kg (1.1 mg/kg) twice daily. After 2 weeks, increase to a maintenance dose of 0.4 mL/kg (1.7 mg/kg) twice daily. ( 2)
- Administer doses at least 9 hours apart during or after feeding. ( 2)
- Readjust dose for changes in the child’s weight. ( 2)
- Monitor heart rate and blood pressure for 2 hours after the first dose or increasing dose. ( 2)
DOSAGE FORMS AND STRENGTHS
Oral solution: 4.28 mg/mL propranolol hydrochloride ( 3)
CONTRAINDICATIONS
- Premature infants with corrected age <5 weeks ( 4 )
- Infants weighing less than 2 kg ( 4)
- Known hypersensitivity to propranolol or excipients ( 4)
- Asthma or history of bronchospasm ( 4, 5.3, 6, 10, 17)
- Bradycardia (<80 beats per minute), greater than first degree heart block, decompensated heart failure ( 4, 5.2, 5.4, 10, 17)
- Blood pressure <50/30 mmHg ( 4, 5.2, 10, 17)
- Pheochromocytoma ( 4)
WARNINGS AND PRECAUTIONS
- Hypoglycemia: Administer during or after feeding. Do not use in patients who are not able to feed or are vomiting ( 4, 5.1, 6, 10, 17)
- Bradycardia and hypotension ( 4, 5.2, 17)
- Bronchospasm: Avoid use in patients with asthma or lower respiratory infection ( 4, 5.3, 6, 10, 17)
- Increased risk of stroke in PHACE syndrome ( 5.5)
ADVERSE REACTIONS
The most common adverse reactions to HEMANGEOL (occurring ≥ 10% of patients) were sleep disorders, aggravated respiratory tract infections, diarrhea, and vomiting. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Pierre Fabre Pharmaceuticals, Inc. at 1-855-PFPHARM (737-4276) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypoglycemia
5.2 Bradycardia and Hypotension
5.3 Bronchospasm
5.4 Cardiac Failure
5.5 Increased Risk of Stroke in PHACE Syndrome
5.6 Hypersensitivity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.4 Pediatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Initiate treatment at ages 5 weeks to 5 months.
The recommended starting dose of HEMANGEOL is 0.15 mL/kg (0.6 mg/kg) (see Table 1) twice daily, taken at least 9 hours apart. After 1 week, increase the daily dose to 0.3 mL/kg (1.1 mg/kg) twice daily. After 2 weeks of treatment, increase the dose to 0.4 mL/kg (1.7 mg/kg) twice daily and maintain this for 6 months. Readjust the dose periodically as the child’s weight increases.
To reduce the risk of hypoglycemia, administer HEMANGEOL orally during or right after a feeding. Skip the dose if the child is not eating or is vomiting [see Warnings and Precautions (5.1)] .
Monitor heart rate and blood pressure for 2 hours after HEMANGEOL initiation or dose increases [see Warnings and Precautions (5.2)].
If hemangiomas recur, treatment may be re-initiated [see Clinical Studies (14)] .
HEMANGEOL is supplied with an oral dosing syringe for administration. Administration directly into the child’s mouth is recommended. Nevertheless, if necessary, the product may be diluted in a small quantity of milk or fruit juice, given in a baby’s bottle.
Table 1. Dose Titration According to Weight
Week 1 Week 2
Week 3 (maintenance)
Weight (kg)
Volume administered
Volume administered
Volume administered
twice a day twice a day twice a day 2 to <2.5
0.3 mL
0.6 mL
0.8 mL
2.5 to <3
0.4 mL
0.8 mL
1 mL
3 to <3.5
0.5 mL
0.9 mL
1.2 mL
3.5 to <4
0.5 mL
1.1 mL
1.4 mL
4 to <4.5
0.6 mL
1.2 mL
1.6 mL
4.5 to <5
0.7 mL
1.4 mL
1.8 mL
5 to <5.5
0.8 mL
1.5 mL
2 mL
5.5 to <6
0.8 mL
1.7 mL
2.2 mL
6 to <6.5
0.9 mL
1.8 mL
2.4 mL
6.5 to <7
1 mL
2 mL
2.6 mL
7 to <7.5
1.1 mL
2.1 mL
2.8 mL
7.5 to <8
1.1 mL
2.3 mL
3 mL
8 to <8.5
1.2 mL
2.4 mL
3.2 mL
8.5 to <9
1.3 mL
2.6 mL
3.4 mL
9 to <9.5
1.4 mL
2.7 mL
3.6 mL
9.5 to <10
1.4 mL
2.9 mL
3.8 mL
10 to <10.5
1.5 mL
3 mL
4 mL
10.5 to <11
1.6 mL
3.2 mL
4.2 mL
11 to <11.5
1.7 mL
3.3 mL
4.4 mL
11.5 to <12
1.7 mL
3.5 mL
4.6 mL
12 to <12.5 1.8 mL 3.6 mL 4.8 mL - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
HEMANGEOL is contraindicated in the following conditions:
- Premature infants with corrected age < 5 weeks
- Infants weighing less than 2 kg
- Known hypersensitivity to propranolol or any of the excipients [see Description (11)]
- Asthma or history of bronchospasm
- Heart rate <80 beats per minute, greater than first degree heart block, or decompensated heart failure
- Blood pressure <50/30 mmHg
- Pheochromocytoma
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypoglycemia
HEMANGEOL prevents the response of endogenous catecholamines to correct hypoglycemia and masks the adrenergic warning signs of hypoglycemia, particularly tachycardia, palpitations and sweating. HEMANGEOL can cause hypoglycemia in children, especially when they are not feeding regularly or are vomiting; withhold the dose under these conditions. Hypoglycemia may present in the form of seizures, lethargy, or coma. If a child has clinical signs of hypoglycemia, discontinue HEMANGEOL and call their health care provider immediately or take the child to the emergency room.
Concomitant treatment with corticosteroids may increase the risk of hypoglycemia [see Drug Interactions (7)] .
5.2 Bradycardia and Hypotension
HEMANGEOL may cause or worsen bradycardia or hypotension. In the studies of HEMANGEOL for infantile hemangioma the mean decrease in heart rate was about 7 bpm with little effect on blood pressure. Monitor heart rate and blood pressure after treatment initiation or increase in dose. Discontinue treatment if severe (<80 beats per minute) or symptomatic bradycardia or hypotension (systolic blood pressure <50 mmHg) occurs.
5.3 Bronchospasm
HEMANGEOL can cause bronchospasm; do not use in patients with asthma or a history of bronchospasm. Interrupt treatment in the event of a lower respiratory tract infection associated with dyspnea and wheezing.
5.4 Cardiac Failure
Sympathetic stimulation supports circulatory function in patients with congestive heart failure, beta blockade may precipitate more severe failure.
5.5 Increased Risk of Stroke in PHACE Syndrome
By dropping blood pressure, HEMANGEOL may increase the risk of stroke in PHACE syndrome patients with severe cerebrovascular anomalies.
Investigate infants with large facial infantile hemangioma for potential arteriopathy associated with PHACE syndrome prior to HEMANGEOL therapy.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Hypoglycemia and related events, like hypoglycemic seizure [see Warnings and Precautions (5.1)].
- Bronchospasm [see Warnings and Precautions (5.3)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical
trials of a drug may not reflect the rates observed in clinical practice.Clinical Trials Experience with HEMANGEOL in Infants with proliferating infantile hemangioma
In clinical trials for proliferating infantile hemangioma, the most frequently reported adverse reactions (>10%) in infants treated with HEMANGEOL were sleep disorders, aggravated respiratory tract infections such as bronchitis and bronchiolitis associated with cough and fever, diarrhea, and vomiting. Adverse reactions led to treatment discontinuation in fewer than 2% of treated patients.Overall, 479 patients in the pooled safety population were exposed to study drug in the clinical study program (456 in placebo-controlled trials). A total of 424 patients were treated with HEMANGEOL at doses 1.2 mg/kg/day or 3.4 mg/kg/day for 3 or 6 months. Of these, 63% of patients were aged 91-150 days and 37% were aged 35-90 days at randomization.
The following table lists according to the dosage the most common adverse reactions (treatment-emergent adverse events with an incidence at least 3% greater on one of the two doses than on placebo).
Table 2. Treatment-emergent adverse events occurring at least 3% more often on HEMANGEOL than on placebo
Reaction
Placebo
N=236HEMANGEOL
1.2 mg/kg/day
N=200HEMANGEOL
3.4 mg/kg/day
N=224Sleep disorder
5.9%
17.5%
16.1%
Bronchitis
4.7
8.0
13.4
Peripheral coldness
0.4
8.0
6.7
Agitation
2.1
8.5
4.5
Diarrhea
1.3
4.5
6.3
Somnolence
0.4
5.0
0.9
Nightmare
1.7
2.0
6.3
Irritability
1.3
5.5
1.3
Decreased appetite
0.4
2.5
3.6
Abdominal pain
0.4
3.5
0.4
The following adverse events have been observed during clinical studies, with an incidence of less than 1%:
Cardiac disorders: Second degree atrioventricular heart block, in a patient with underlying conduction disorder, required definitive treatment discontinuation [see Warnings and Precautions(5.4)] .
Skin and subcutaneous tissue disorders: Urticaria, alopecia
Investigations: Decreased blood glucose, decreased heart rate
Compassionate Use Program
More than 600 infants received HEMANGEOL in a compassionate use program (CUP). Mean age at treatment initiation was 3.6 months. Mean dose of HEMANGEOL was 2.2 mg/kg/day and mean treatment duration was 7.1 months.
The adverse reactions reported in the CUP were similar to the ADRs observed during clinical trials but some were more severe.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of propranolol. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
These adverse reactions are as follows:
Blood and lymphatic system disorders:Agranulocytosis
Psychiatric disorders: Hallucination
Skin and subcutaneous tissues disorders: Purpura, dermatitis psoriasform
-
7 DRUG INTERACTIONS
In the absence of specific studies in children, the drug interactions with propranolol are those known in adults. Consider both the infant’s medications and those of a nursing mother.
Pharmacokinetic drug interactions
Impact of co-administered drugs on propranolol: CYP2D6, CYP1A2 or CYP2C19 inhibitors increase propranolol plasma concentration. CYP1A2 inducers (phenytoin, phenobarbital) or CYP2C19 inducers (rifampin) decrease propranolol plasma concentration when co-administered.
Pharmacodynamic drug interactions
Corticosteroids: Patients on corticosteroids may be at increased risk of hypoglycemia because of loss of the counter-regulatory cortisol response; monitor patients for signs of hypoglycemia.
-
8 USE IN SPECIFIC POPULATIONS
8.4 Pediatric Use
Of 460 infants with proliferating infantile hemangioma requiring systemic therapy who were treated with HEMANGEOL starting at 5 weeks to 5 months of age, 60% had complete or nearly complete resolution of their hemangioma at Week 24 [see Clinical Studies (14)].
Safety and effectiveness for infantile hemangioma have not been established in pediatric patients greater than 1 year of age.
-
10 OVERDOSAGE
Few cases of propranolol overdose were reported. For a single intake, the maximum dose was 20 mg/kg. Symptomatic cases featured hypotension, hypoglycemic seizure, and restlessness/euphory/insomnia; for most cases, propranolol was maintained or reintroduced.
The toxicity of beta-blockers is an extension of their therapeutic effects:
- Cardiac symptoms of mild to moderate poisoning are decreased heart rate and hypotension. Atrioventricular blocks, intraventricular conduction delays, and congestive heart failure can occur with more severe poisoning.
- Bronchospasm may develop particularly in patients with asthma.
- Hypoglycemia may develop and manifestations of hypoglycemia (tremor, tachycardia) may be masked by other clinical effects of beta-blocker toxicity.
Support and treatment: Place the patient on a cardiac monitor, and monitor vital signs, mental status and blood glucose. Give intravenous fluids for hypotension and atropine for bradycardia. Glucagon then catecholamines should be considered if the patient does not respond appropriately to IV fluid. Isoproterenol and aminophylline may be used for bronchospasm.
Propranolol is not dialyzable.
-
11 DESCRIPTION
HEMANGEOL is an oral solution of propranolol that is alcohol free, paraben free and sugar free. Each mL of HEMANGEOL contains 4.28 mg of propranolol hydrochloride, USP equivalent to 3.75 mg of propranolol.
Propranolol hydrochloride is a synthetic beta-adrenergic receptor blocking agent chemically described as (2RS)1-[(1-methylethyl)amino]-3-(naphthalene-1-yloxy)-propan-2-ol hydrochloride. Its structural formula is shown in Figure 1:
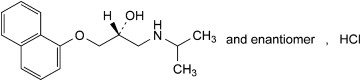
Figure 1. Propranolol HCl Structure
Molecular formula: C 16H 21NO 2-HCl
Propranolol hydrochloride is a stable, white, crystalline solid with a molecular weight of 295.8. It is readily soluble in water and ethanol.
HEMANGEOL contains the following inactive ingredients: strawberry/vanilla flavorings, hydroxyethylcellulose, saccharin sodium, citric acid monohydrate, and water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of HEMANGEOL’s effects on infantile hemangiomas is not well understood.
12.2 Pharmacodynamics
Propranolol is a nonselective beta-adrenergic receptor blocking agent possessing no other autonomic nervous system activity. It specifically competes with beta-adrenergic receptor stimulating agents for available receptor sites. When access to beta-receptor sites is blocked by propranolol, chronotropic, inotropic, and vasodilator responses to beta-adrenergic stimulation are decreased proportionately.
Propranolol selectively blocks beta-adrenergic receptors, leaving alpha-adrenergic responses intact. There are two well-characterized subtypes of beta receptors (beta1 and beta2); propranolol interacts with both subtypes equally.
Beta1-adrenergic receptors are found primarily in the heart. Blockade of cardiac beta1-adrenergic receptors leads to a decrease in the activity of both normal and ectopic pacemaker cells and a decrease in A-V nodal conduction velocity. Blockade of cardiac beta1-adrenergic receptors also decreases the myocardial force of contraction and may provoke cardiac decompensation in patients with minimal cardiac reserve.
Beta2-adrenergic receptors are found predominantly in smooth muscle-vascular, bronchial, gastrointestinal and genitourinary. Blockade of these receptors results in constriction. Propranolol’s beta-blocking effects are attributable to its S(-) enantiomer.
Pharmacodynamic drug interactions
Alpha blockers: Co-administration of beta-blockers with alpha blockers (prazosin) has been associated with prolongation of first dose hypotension and syncope.
Antidepressants: The hypotensive effect of MAO inhibitors and tricyclic antidepressants is exacerbated when administered with beta-blockers.
Nonsteroidal anti-inflammatory drugs: Nonsteroidal anti-inflammatory drugs (NSAIDs) may attenuate the antihypertensive effect of beta-adrenoreceptor blocking agents. Monitor blood pressure.
12.3 Pharmacokinetics
Adults
Absorption: Propranolol is almost completely absorbed after oral administration. However, it undergoes an extensive first-pass metabolism by the liver and on average; only about 25% of propranolol reaches the systemic circulation. Peak plasma concentrations occur about 1 to 4 hours after an oral dose. Administration of protein-rich foods increases the bioavailability of propranolol by about 50% with no change in time to peak concentration.
Propranolol is a substrate for the intestinal efflux transporter, P-glycoprotein (P-gp). However, studies suggest that P-gp is not dose-limiting for intestinal absorption of propranolol in the usual therapeutic dose range.
Distribution: Approximately 90% of circulating propranolol is bound to plasma proteins (albumin and alpha1 acid glycoprotein). The volume of distribution of propranolol is approximately 4 L/kg. Propranolol crosses the blood-brain barrier and the placenta, and is distributed into breast milk.
Propranolol is extensively metabolized with most metabolites appearing in the urine.
Metabolism: Propranolol is metabolized through three primary routes: aromatic hydroxylation (mainly 4-hydroxylation), N-dealkylation followed by further side-chain oxidation, and direct glucuronidation. The percentage contributions of these routes to total metabolism are 42%, 41% and 17%, respectively, but with considerable variability between individuals. The four major final metabolites are propranolol glucuronide, naphthyloxylactic acid and glucuronic acid, and sulfate conjugates of 4-hydroxy propranolol. In vitro studies indicated that CYP2D6 (aromatic hydroxylation), CYP1A2 (chain oxidation) and to a less extent CYP2C19 were involved in propranolol metabolism.
In healthy subjects, no difference was observed between CYP2D6 extensive metabolizers (EMs) and poor metabolizers (PMs) with respect to oral clearance or elimination half-life.
Elimination:The plasma half-life of propranolol ranges from 3 to 6 hours. Less than 1% of a dose is excreted as unchanged drug in the urine.
Infants
The pharmacokinetics of propranolol and 4-OH-propranolol were evaluated in a multiple dose 12 week study conducted in 23 male and female infants 35 to 150 days of age with hemangioma. The infants were stratified by age (35 to 90 days and 91 to 150 days). The starting dose was 1.2 mg/kg/day which was titrated to the target dose of 3.4 mg/kg/day in 1.1 mg/kg/day increments at weekly intervals. At steady state, following administration of 3.4 mg/kg/day twice daily, peak plasma propranolol concentrations were observed within 2 hours of oral administration. Clearance of propranolol in infants was similar across the age range studied (2.7 (SD=0.03) L/h/kg in infants <90 days of age and 3.3 (SD=0.35) L/h/kg in infants >90 days of age) and to that in adults when adjusted by body weight. The median elimination half-life of propranolol was about 3.5 hours. Plasma propranolol concentrations approximate a dose proportional increase in the dose range of 1.2 mg/kg/day to 3.4 mg/kg/day.
Plasma concentration of 4-OH-propranolol, the main metabolite, was about 5% of total plasma exposure of propranolol.
Sex
There is no known dependence of pharmacokinetics of propranolol by sex in infants.
Race
There is little information on dependence of pharmacokinetics of propranolol by race in infants.
A study conducted in 12 Caucasian and 13 African-American adult male subjects taking propranolol, showed that at steady state, the clearance of R(+)- and S(-)-propranolol were about 76% and 53% higher in African-Americans than in Caucasians, respectively.
Chinese adult subjects had a greater proportion (18% to 45% higher) of unbound propranolol in plasma compared to Caucasians, which was associated with a lower plasma concentration of alpha1 acid glycoprotein.
Drug Interaction Studies
Impact of propranolol on co-administered drugs: The effect of propranolol on plasma concentration of co-administered drug is presented in the table below.
Table 3. Effect of propranolol on co-administered drugs
Co-administered drug
Effect on plasma concentration of co-administered drug
Amide anesthetics (lidocaine, bupivacaine, mepivacaine)
Increase
Warfarin
Increase
Propafenone
Increase > 200 %
Nifedipine
Increase 80 %
Verapamil
No change
Pravastatin, lovastatin
Decrease 20%
Fluvastatin
No change
Zolmitriptan
Increase 60 %
Rizatriptan
Increase 80 %
Thioridazine
Increase 370 %
Diazepam
Increase
Oxazepam, triazolam, lorazepam, alprazolam
No change
Theophylline
Increase 70 %
Impact of co-administered drugs on propranolol: The effect of co-administered drugs on propranolol plasma concentration is presented in the table below.
Table 4. Effect of co-administered drugs on propranolol
Co-administered drug
Effect on propranolol plasma concentration
CYP2D6, CYP1A2 or CYP2C19 inhibitors
Increase
CYP1A2 or CYP2C19 inducers
Decrease
Quinidine
Increase > 200 %
Nisoldipine
Increase 50 %
Nicardipine
Increase 80 %
Chlorpromazine
Increase 70 %
Cimetidine
Increase 50 %
Cholestyramine, colestipol
Decrease 50 %
Alcohol
Increase (acute use), decrease (chronic use)
Diazepam
No change
Verapamil
No change
Metoclopramide
No change
Ranitidine
No change
Lansoprazole
No change
Omeprazole
No change
Propafenone
Increase 200 %
Aluminum hydroxide
Decrease 50 %
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility
In studies of mice and rats fed propranolol hydrochloride for up to 18 months at doses of up to 150 mg/kg/day, there was no evidence of drug-related tumorigenesis. On a body surface area basis, this dose in the mouse and rat is about 3 and 7 times, respectively, the MRHD of 3.4 mg/kg/day propranolol hydrochloride in children.
Based on differing results from bacterial reverse mutation (Ames) tests performed by different laboratories, there is equivocal evidence for mutagenicity in one strain ( S. typhimurium strain TA 1538).
In a study in which both male and female rats were exposed to propranolol hydrochloride via diet at concentrations of up to 0.05% (about 50 mg/kg or less than the MRHD of 640 mg propranolol hydrochloride in adults) started from 60 days prior to mating and throughout pregnancy and lactation for two generations, there were no effects on fertility. The potential effects of propranolol hydrochloride on fertility of juvenile rats were evaluated following daily oral administration from post-natal Day 4 (PND 4) to PND 21 at dose-levels of 0, 11.4, 22.8 or 45.6 mg/kg/day. No propranolol related effects on reproductive parameters or reproductive development were observed up to the highest dose level of 45.6 mg/kg/day, a dose that represents a systemic exposure of 3 times that seen in children at the MRHD.
13.2 Animal Toxicology and/or Pharmacology
This study in juvenile rats with propranolol hydrochloride described above was intended to cover the period of development corresponding to infancy, childhood and adolescence. Neurologic effects including hypoactivity and delayed air righting reflex, increased germinal centers of lymph nodes, and increased white blood cells and lymphocytes were seen at a propranolol hydrochloride dose 45.6 mg/kg/day that represents a systemic exposure of 3 times that seen in children at the MRHD. Body weights were transiently decreased, and transient decreases in urine volume were associated with higher incidences of minimal renal cysts and dilation of kidney tubules at doses about equal to the MRHD in children.
-
14 CLINICAL STUDIES
A randomized, double-blind study in 460 infants, aged 35 days to 5 months at inclusion, with proliferating infantile hemangiomas (IH) requiring systemic therapy (excluding life-threatening IH, function-threatening IH, and ulcerated IH with pain and lack of response to simple wound care measures) compared four regimens of HEMANGEOL (1.2 or 3.4 mg/kg/day in twice daily divided doses for 3 or 6 months; N=99-103 per group) to placebo (N=55). Clinical efficacy was evaluated by counting complete or nearly complete resolution of the target hemangioma, which was evaluated by blinded centralized independent assessments of photographs at Week 24 compared to baseline.
Demographic patient characteristics and hemangioma characteristics were similar among the five regimens. For the whole population, 29% were male, 37% were in the lower age group (35-90 days), and 72% were Caucasian. Overall, 70% had a target hemangioma on the head, most commonly cheek (13%) and forehead (11%).
The main reason for treatment discontinuation was the treatment inefficacy, which happened in 58% of patients randomized to placebo, 25-30% of patients randomized to HEMANGEOL for 3 months (mainly after the switch to placebo), and 7-9% of patients randomized to HEMANGEOL for 6 months.
Overall, 2 out of 55 patients (4%) in the placebo arm and 61 out of 101 patients (60%) on HEMANGEOL 3.4 mg/kg/day for 6 months had complete or nearly complete resolution of their hemangioma at Week 24 (p <0.0001).
There were no significant differences in response by age (35-90 days / 91-150 days), sex, or hemangioma site. There were too few non-Caucasians to assess differences in effects by race.
Of patients on HEMANGEOL 3.4 mg/kg/day for 6 months who were considered successes, 10% required retreatment for recurrence of hemangiomas.
A second uncontrolled study in 23 patients with proliferating IH included function-threatening IH, IH in certain anatomic locations that often leave permanent scars or deformity, large facial IH, smaller IH in exposed areas, severe ulcerated IH, pedunculated IH. Target lesions resolved in 36% of patients by 3 months.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
HEMANGEOL is supplied as an oral solution. Each 1 mL contains 4.28 mg propranolol hydrochloride. HEMANGEOL is supplied in a carton containing one 120 mL bottle with syringe adapter and one 5 mL oral dosing syringe.
NDC: 64370-375-01 Bottle 120 mL
16.2 Storage and Handling
Store at 25ºC (77ºF); excursions permitted from 15º to 30ºC (59º to 86ºF). [See USP Controlled Room Temperature.]
Do not freeze.
Do not shake the bottle before use.
Dispense in original container with enclosed oral dosing syringe.
The product can be kept for 2 months after first opening.
See instructions for using enclosed oral dosing syringe.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide and Instructions for Use).
Patient advice
Advise parents or caregivers to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Instructions for using oral dosing syringe
Instruct parents or caregivers on use of the oral dosing syringe.
Risk of hypoglycemia
Inform parents or caregivers that there is a risk of hypoglycemia when HEMANGEOL is given to infants who are not feeding regularly or who are vomiting. Instruct them to skip dosing under such conditions.
Instruct parents or caregivers how to recognize the signs of hypoglycemia. Tell them to discontinue HEMANGEOL and call their health care provider immediately or take the child to the emergency room in case of suspected hypoglycemia.
Cardiovascular risks
Advise parents or caregivers that there is a potential risk for bradycardia, aggravation of pre-existing conduction disorders, and hypotension associated with the use of HEMANGEOL. Instruct them to contact their healthcare provider in case of fatigue, pallor, slow or uneven heart beats, peripheral coldness or fainting.
Respiratory risks
Inform parents or caregivers that HEMANGEOL carries risk of bronchospasm or exacerbation of lower respiratory tract infections. Instruct them to contact their healthcare provider or go to the nearest hospital emergency room if their child has breathing problems or wheezing during treatment with HEMANGEOL.
Other risks
Inform parents or caregivers that changes in sleep patterns may occur during HEMANGEOL therapy.
Ask parents or caregivers to tell you all the medications they are administering to their child including prescription and over the counter medicines, vitamins, and herbal supplements. Ask breastfeeding mothers to tell you all the medications they are currently taking, as these may pass into the milk.
-
MEDICATION GUIDE
MEDICATION GUIDE
HEMANGEOL ® (he-man je-ohl)
(propranolol hydrochloride oral solution)
What is the most important information I should know about HEMANGEOL?
HEMANGEOL can cause serious side effects, including:
- Low blood sugar (hypoglycemia), especially if your child is not taking feedings, or is vomiting. HEMANGEOL may make it more difficult to recognize the signs and symptoms of low blood sugar in your child.
To help reduce the risk of low blood sugar with HEMANGEOL:
- Give HEMANGEOL during or shortly after feeding your child.
- Feed your child regularly during treatment. Tell your doctor if your child has a poor appetite.
- If your child is not taking feedings, for example, due to an illness or vomiting, do not give HEMANGEOL until your child is taking feedings normally again.
If your child has any of the signs or symptoms of low blood sugar listed below during treatment with HEMANGEOL, stop giving your child HEMANGEOL and call your doctor or go to the nearest emergency room right away.
Signs or symptoms of low blood sugar include: pale, blue or purple skin color, sweating, irritability, crying for no apparent reason, irregular or fast heartbeat, poor feeding, low body temperature, unusual sleepiness, seizures, breathing stops for short periods of time, and loss of consciousness.
What is HEMANGEOL?
HEMANGEOL is a prescription medicine used to treat proliferating infantile hemangioma that requires treatment with a medicine that spreads throughout the body.
Who should not take HEMANGEOL?
Do not give HEMANGEOL to your child if your child:
- was born prematurely and has not reached the corrected age of 5 weeks
- weighs less than 4 ½ pounds
- is allergic to propranolol or any of the other ingredients in HEMANGEOL. See the end of this Medication Guide for a list of ingredients in HEMANGEOL
- has asthma or a history of breathing problems
- has a heart problem, slow heart rate (less than 80 heart beats per minute), very low blood pressure
- is at risk for low blood sugar, for example is vomiting or unable to take feedings
- has high blood pressure caused by a tumor on the adrenal gland, called “pheochromocytoma”
What should I tell my doctor before giving my child HEMANGEOL?
Before you start giving HEMANGEOL to your child,tell your doctor about all of your child’s medical conditions.
Tell your doctor about all of the medicines that your child takes, including prescription and over-the-counter medicines, vitamins, and herbal supplements. HEMANGEOL and some medicines may interact with each other and cause serious side effects. Especially tell your doctor if your child takes a steroid medicine. Taking a steroid medicine during treatment with HEMANGEOL may increase your child’s risk of low blood sugar.
If you are breastfeeding your child, it is important to tell your doctor about all the medicines you take. Certain medicines may pass to your child through your breast-milk and interact with HEMANGEOL. Your doctor should tell you if you should stop breastfeeding.
How should I give HEMANGEOL to my child?
Follow the detailed Instructions for Use that come with HEMANGEOL for information about the correct way to prepare and give a dose of HEMANGEOL.
- Give HEMANGEOL to your child exactly as your doctor tells you.
- Your doctor may change the dose until it is right for your child, and as your child’s weight changes.
- Always give HEMANGEOL with a feeding or right away after a feeding.
- HEMANGEOL is given 2 times each day, at least 9 hours apart.
- If your child spits up a dose or if you are not sure your child got all of the medicine, do not give another dose. Wait until the next scheduled dose.
What are the possible side effects of HEMANGEOL?
See “ What is the most important information I should know about HEMANGEOL?”
HEMANGEOL can cause serious side effects, including:
- New or worsening slow heart rate (bradycardia) or low blood pressure (hypotension). Call your doctor if your child has any of these symptoms: pale skin color, slow or uneven heartbeats, arms or legs feel cold, blue or purple skin color, or fainting.
- Breathing problems or wheezing. HEMANGEOL can cause spasms of your child’s airway. Call your doctor or go to the nearest hospital emergency room if your child has breathing problems or wheezing during treatment with HEMANGEOL.
- Stroke. HEMANGEOL may increase the risk of stroke in certain children who have severe problems with the blood vessels in their brain, particularly if your child has a large hemangioma that affects the face or head.
The most common side effects include: sleep problems, worsening respiratory tract infections, diarrhea, and vomiting.
These are not all the possible side effects of HEMANGEOL. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store HEMANGEOL?
- Store HEMANGEOL at room temperature between 68 o F to 77 o F (20 o C to 25 o C). Do not freeze. Do not shake before use.
- Safely throw away any opened bottle of HEMANGEOL after 2 months, even if there is medicine left in the bottle.
Keep HEMANGEOL and all medicines out of the reach of children.
General information about the safe and effective use of HEMANGEOL.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or doctor for information about HEMANGEOL that is written for health professionals. Do not use HEMANGEOL for a condition for which it was not prescribed. Do not give HEMANGEOL to other people, even if they have the same symptoms your child has. It may harm them.
What are the ingredients in HEMANGEOL?
Active ingredient: propranolol hydrochloride
Inactive ingredients: strawberry flavor, vanilla flavor, hydroxyethylcellulose, saccharin sodium, citric acid monohydrate, and water.
Manufactured for: Pierre Fabre Pharmaceuticals, Inc., Parsippany, NJ 07054
For more information, call 1-855-PFPHARM (737-4276)
This Medication Guide has been approved by the U.S. Food and Drug Administration Issued: Jun 2016
-
INSTRUCTIONS FOR USE
Instructions for Use
HEMANGEOL ® (he-man je-ohl)
(propranolol hydrochloride oral solution)
Read these Instructions for Use before giving a dose of HEMANGEOL to your child for the first time and each time you get a refill. There may be new information. Your doctor or pharmacist should show you how to correctly measure and give a dose of HEMANGEOL to your child before you give it for the first time.
Important: Read the Medication Guide that comes with HEMANGEOL.
- To reduce the risk of your child getting low blood sugar (hypoglycemia), you must give HEMANGEOL either during a feeding or right away after a feeding.
- Do not give a dose of HEMANGEOL if your child is vomiting, is not taking feedings, or is showing signs or symptoms of low blood sugar.
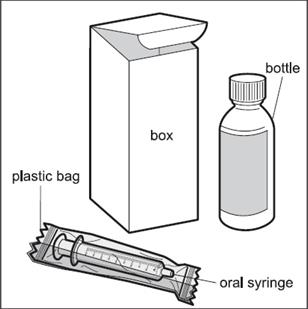
When you get HEMANGEOL from your doctor or pharmacist, you will receive a box that contains the supplies needed to give HEMANGEOL to your child, including:
- One glass bottle of HEMANGEOL
- One 5 mL oral dosing syringe (inside a plastic bag) that is marked to help you correctly measure a dose of HEMANGEOL ( See Figure A). If the carton does not contain the oral dosing syringe, ask your pharmacist to give you an oral dosing syringe that can be used to measure HEMANGEOL.
Figure A
Preparing to give your child a dose of HEMANGEOL:
Step 1. Place your box of supplies on a clean flat work surface, such as a table.
Step 2. Remove the HEMANGEOL bottle and oral dosing syringe from the box ( See Figure A above). Do not shake the bottle before use. Keep the box for storage.
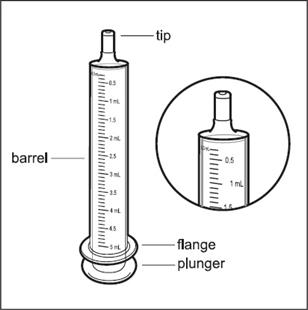
Step 3. Remove the oral dosing syringe from the plastic bag. Safely throw the plastic bag away. The barrel of the syringe has markings in milliliters (mL). Look at the markings on the barrel of the oral dosing syringe and find the mL marking that matches the HEMANGEOL dose in mL prescribed by your doctor ( See Figure B).
Figure B
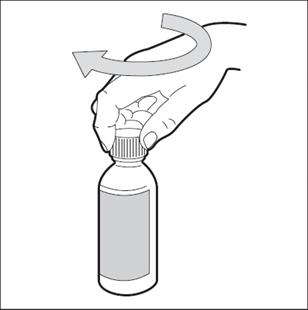
Step 4. Open the bottle of HEMANGEOL by pushing down on the plastic cap while turning the cap to the left ( See Figure C).
- Write down on the box the date when you first open the bottle.
Figure C
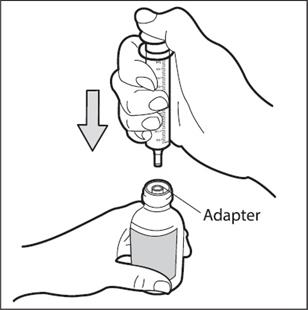
Step 5. Place the bottle on your work surface. Use one hand to hold the bottle upright.Use your other hand to insert the tip of the oral dosing syringe into the syringe adapter at the top of the bottle. Push the plunger all the way down ( See Figure D).
- Do not remove the syringe adapter. If the syringe adapter is missing talk to your pharmacist.
Figure D
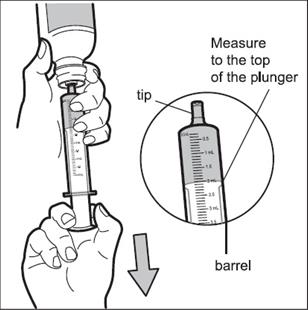
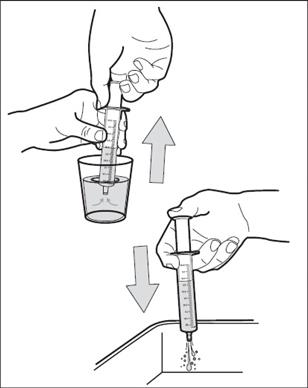
Step 6: Use one hand to hold the oral dosing syringe in place. With your other hand, turn the bottle upside down. Pull back on the plunger until the top of the plunger lines up with the marking on the barrel of the syringe that matches the dose of HEMANGEOL prescribed by your doctor ( See Figure E). Your child’s dose may be different than the dose shown in Figure E.
Figure E
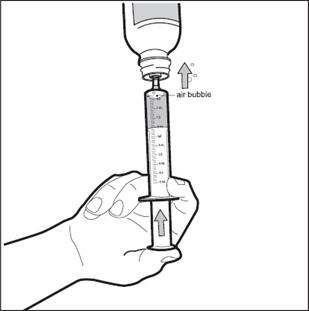
Step 7: Check for air bubbles in the oral dosing syringe. If you see air bubbles, push up on the plunger towards the bottle just enough to remove any large air bubbles and then pull back to the measured dose ( See Figure F).
Figure F
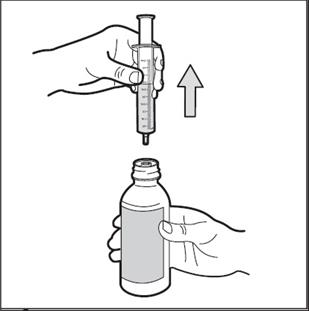
Step 8. Turn bottle upright again and place it in on your work surface. Remove the oral dosing syringe from the bottle ( See Figure G). Do not push the plunger in during this step. The syringe adapter should stay attached to the bottle.
Figure G
Giving your child a dose of HEMANGEOL:
Step 9. Slowly squirt HEMANGEOL into your child’s mouth after placing the oral dosing syringe against the inside of the cheek ( See Figure H).
- Keep your child in an upright position for a few minutes right after giving a dose of HEMANGEOL.
Figure H
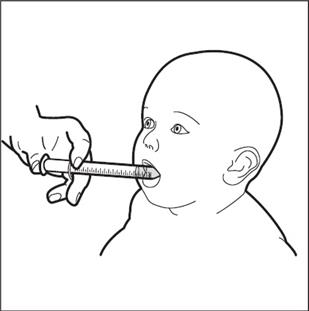
- If needed, you can dilute the dose of HEMANGEOL in a small amount of milk or fruit juice and give it to your child in a baby’s bottle. If your child spits up a dose or if you are not sure your child got all of the medicine, do not give another dose. Wait until the next scheduled dose.
Step 10. Replace the plastic cap on the bottle. Close the bottle by turning the plastic cap to the right ( See Figure I).
Figure I
Cleaning the oral dosing syringe:
Step 11: Clean the oral dosing syringe after each use by rinsing with clean tap water ( See Figure J).
- Do not take apart the oral dosing syringe.
- Do not use any soap or alcohol based product to clean. Wipe the outside dry.
- Do not put the oral dosing syringe through a sterilizer or dishwasher.
Figure J
Step 12: Place the bottle and the oral dosing syringe in the box.
How should I store HEMANGEOL?
- When not in use, keep the bottle of HEMANGEOL and the oral dosing syringe in the box it comes in.
- Store HEMANGEOL at room temperature, between 68 o F to 77 o F (20 o C to 25 o C). Do not freeze.
- Safely throw away any opened bottle of HEMANGEOL after 2 months, even if there is medicine left in the bottle.
Keep HEMANGEOL and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Pierre Fabre Pharmaceuticals, Inc.
Parsippany, NJ 07054
-
PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL - 120 mL Bottle Label
NDC: 64370-375-01
Hemangeol ®
(propranolol hydrochloride)
oral solution
4.28 mg/mL
Dispense with enclosed Medication Guide
Rx only
120 mL
PRINCIPAL DISPLAY PANEL - 120 mL Carton Label
NDC: 64370-375-01
Hemangeol ®
(propranolol hydrochloride)
oral solution
4.28 mg/mL
Pharmacist: Dispense the enclosed Medication Guide to each patient
Rx only
120 mL
-
INGREDIENTS AND APPEARANCE
HEMANGEOL
propranolol hydrochloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 64370-375 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPRANOLOL HYDROCHLORIDE (UNII: F8A3652H1V) (PROPRANOLOL - UNII:9Y8NXQ24VQ) PROPRANOLOL HYDROCHLORIDE 4.28 mg in 1 mL Product Characteristics Color Score Shape Size Flavor VANILLA, STRAWBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64370-375-01 1 in 1 CARTON 04/14/2014 1 120 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 2 NDC: 64370-375-50 1 in 1 CARTON 04/14/2014 2 50 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205410 04/14/2014 Labeler - Pierre Fabre Pharmaceuticals, Inc. (968997101) Registrant - Pierre Fabre Pharmaceuticals, Inc. (968997101)
Trademark Results [HEMANGEOL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HEMANGEOL 86183672 4611446 Live/Registered |
PIERRE FABRE DERMATOLOGIE 2014-02-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.