Cephalexin by Advanced Rx Pharmacy of Tennessee, LLC CEPHALEXIN capsule
Cephalexin by
Drug Labeling and Warnings
Cephalexin by is a Prescription medication manufactured, distributed, or labeled by Advanced Rx Pharmacy of Tennessee, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
1. Indications and Usage Section
1 INDICATIONS & USAGE
1.1 Respiratory Tract Infections
Cephalexin is indicated for the treatment of respiratory tract infections caused by susceptible isolates of Streptococcus pneumoniae and Streptococcus pyogenes.
1.2 Otitis Media
Cephalexin is indicated for the treatment of otitis media caused by susceptible isolates of Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pyogenes, and Moraxella catarrhalis.
1.3 Skin and Skin Structure Infections
Cephalexin is indicated for the treatment of skin and skin structure infections caused by susceptible isolates of the following Gram-positive bacteria: Staphylococcus aureus and Streptococcus pyogenes.
1.4 Bone Infections
Cephalexin is indicated for the treatment of bone infections caused by susceptible isolates of Staphylococcus aureus and Proteus mirabilis.
1.5 Genitourinary Tract Infections
Cephalexin is indicated for the treatment of genitourinary tract infections, including acute prostatitis, caused by susceptible isolates of Escherichia coli, Proteus mirabilis, and Klebsiella pneumoniae.
1.6 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cephalexin and other antibacterial drugs, Cephalexin should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information is available, this information should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2. Dosage and Administration Section
2 DOSAGE & ADMINISTRATION
2.1 Adults and Pediatric Patients at Least 15 Years of Age
The usual dose of oral Cephalexin capsule, USP is 250 mg every 6 hours, but a dose of 500 mg every 12 hours may be administered. Treatment is administered for 7 to 14 days.
For more severe infections larger doses of oral Cephalexin capsules, USP may be needed, up to 4 grams daily in two to four equally divided doses.
2.2 Pediatric Patients (over 1 year of age)
The recommended total daily dose of oral Cephalexin capsules, USP for pediatric patients is 25 to 50 mg/kg given in equally divided doses for 7 to 14 days. In the treatment of β-hemolytic streptococcal infections, duration of at least 10 days is recommended. In severe infections, a total daily dose of 50 to 100 mg/kg may be administered in equally divided doses.
For the treatment of otitis media, the recommended daily dose is 75 to 100 mg/kg given in equally divided doses.
2.3 Dosage Adjustments in Adult and Pediatric Patients at Least 15 Years of Age with Renal Impairment
Administer the following dosing regimens for Cephalexin capsules, USP to patients with renal impairment [see Warnings and Precautions (5.4) and Use in Specific Populations (8.6 )].
Table 1. Recommended Dose Regimen for Patients with Renal Impairment
Renal function Dose regimen recommendation
Creatinine clearance >60mL/min. No dose adjustment
Creatinine clearance 30 to 59 mL / min
No dose adjustment; maximum daily dose should not exceed 1 g
Creatinine clearance 15 to 29 mL / min
250 mg, every 8 hours or every 12 hours
Creatinine clearance 5 to 14 mL / min not yet on dialysis*
250 mg, every 24 hours
Creatinine clearance 1 to 4 mL / min not yet on dialysis*
250 mg, every 48 hours or every 60 hours*There is insufficient information to make dose adjustment recommendations in patients on hemodialysis.
-
3. Dosage Forms and Strengths
250 mg capsules: a white to off white powder filled into size 2 capsules (dark green cap and dark green body) that are imprinted with “220” on the both cap and body in edible black ink.
500 mg capsules: a white to off white powder filled into size 0 capsules (light green cap and light green body) that are imprinted with “219” on the both cap and body in edible black ink.
333 mg capsules: a white to off white powder filled into size 1 capsules (light green cap and light green body) that are imprinted “CEP” on cap and “333” on body in edible black ink.
750 mg capsules: a white to off white powder filled into size '00 Elongated' capsules (dark green cap and dark green body) that are imprinted “CEP” on cap and “750” on body in edible white ink.
- 4. Contraindications
-
5. Warnings and Precautions
5.1 Hypersensitivity Reactions
Allergic reactions in the form of rash, urticaria, angioedema, anaphylaxis, erythema multiforme, Stevens- Johnson syndrome, or toxic epidermal necrolysis have been reported with the use of cephalexin. Before therapy with cephalexin is instituted, inquire whether the patient has a history of hypersensitivity reactions to cephalexin, cephalosporins, penicillins, or other drugs. Cross-hypersensitivity among beta-lactam antibacterial drugs may occur in up to 10% of patients with a history of penicillin allergy.
If an allergic reaction to cephalexin occurs, discontinue the drug and institute appropriate treatment.5.2 Clostridium difficile-Associated Diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cephalexin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B, which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.3 Direct Coombs’ Test Seroconversion
Positive direct Coombs’ tests have been reported during treatment with the cephalosporin antibacterial drugs including cephalexin. Acute intravascular hemolysis induced by cephalexin therapy has been reported. If anemia develops during or after cephalexin therapy, perform a diagnostic work-up for drug-induced hemolytic anemia, discontinue cephalexin and institute appropriate therapy.
5.4 Seizure Potential
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced. If seizures occur, discontinue cephalexin. Anticonvulsant therapy can be given if clinically indicated.
5.5 Prolonged Prothrombin Time
Cephalosporins may be associated with prolonged prothrombin time. Those at risk include patients with renal or hepatic impairment, or poor nutritional state, as well as patients receiving a protracted course of antibacterial therapy, and patients receiving anticoagulant therapy. Monitor prothrombin time in patients at risk and manage as indicated.
5.6 Development of Drug-Resistant Bacteria
Prescribing cephalexin in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Prolonged use of cephalexin may result in the overgrowth of nonsusceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
-
6. Adverse Reactions
The following serious events are described in greater detail in the Warning and Precautions section:
Hypersensitivity reactions [see Warning and Precautions (5.1 )]
Clostridium difficile-associated diarrhea [see Warnings and Precautions (5.2 )]
Direct Coombs’ Test Seroconversion [see Warnings and Precautions (5.3 )]
Seizure Potential [see Warnings and Precautions (5.4 )]
Effect on Prothrombin Activity [see Warnings and Precautions (5.5 )]
Development of Drug-Resistant Bacteria [see Warnings and Precautions (5.6 )]6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice
In clinical trials, the most frequent adverse reaction was diarrhea. Nausea and vomiting, dyspepsia, gastritis, and abdominal pain have also occurred. As with penicillins and other cephalosporins, transient hepatitis and cholestatic jaundice have been reported.
Other reactions have included hypersensitivity reactions, genital and anal pruritus, genital candidiasis, vaginitis and vaginal discharge, dizziness, fatigue, headache, agitation, confusion, hallucinations, arthralgia, arthritis, and joint disorder. Reversible interstitial nephritis has been reported. Eosinophilia, neutropenia, thrombocytopenia, hemolytic anemia, and slight elevations in aspartate transaminase (AST) and alanine transaminase (ALT) have been reported.
In addition to the adverse reactions listed above that have been observed in patients treated with cephalexin, the following adverse reactions and other altered laboratory tests have been reported for cephalosporin class antibacterial drugs:
Other Adverse Reactions: Fever, colitis, aplastic anemia, hemorrhage, renal dysfunction, and toxic nephropathy.
Altered Laboratory Tests: Prolonged prothrombin time, increased blood urea nitrogen (BUN), increased creatinine, elevated alkaline phosphatase, elevated bilirubin, elevated lactate dehydrogenase (LDH), pancytopenia, leukopenia, and agranulocytosis.
-
7. Drug Interactions
7.1 Metformin
Administration of cephalexin with metformin results in increased plasma metformin concentrations and decreased renal clearance of metformin.
Careful patient monitoring and dose adjustment of metformin is recommended in patients concomitantly taking cephalexin and metformin [see Clinical Pharmacology (12.3)]
7.2 Probenecid
The renal excretion of cephalexin is inhibited by probenecid. Co-administration of probenecid with cephalexin is not recommended.
7.3 Interaction with Laboratory or Diagnostic Testing
A false-positive reaction may occur when testing for the presence of glucose in the urine using Benedict’s solution or Fehling’s solution.
-
8. Use in Specific Populations
8.1 Pregnancy
Risk Summary
Available data from published epidemiologic studies and pharmacovigilance case reports over several decades with cephalosporin use, including cephalexin use in pregnant women have not established drug-associated risks of major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data).
Animal reproduction studies with mice and rats using oral doses of cephalexin that are 0.6- and 1.2-times the maximum recommended human dose (MRHD) based on body surface area during organogenesis revealed no evidence of harm to the fetus (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
While available studies cannot definitively establish the absence of risk, published data from epidemiologic studies and postmarketing case reports over several decades have not identified a consistent association with cephalosporin use, including cephalexin, during pregnancy, and major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Available studies have methodologic limitations, including small sample size, retrospective data collection, and inconsistent comparator groups.
Animal Data
In animal reproduction studies, pregnant mice and rats administered oral cephalexin doses of 250 or 500 mg/kg/day (approximately 0.6 and 1.2 times the MRHD) based on body surface area, respectively during the period of organogenesis showed no adverse effects on embryofetal development.
In a pre-and post-natal developmental toxicity study, pregnant rats that received oral doses of 250 or 500 mg/kg/day of cephalexin from Day 15 of pregnancy to litter Day 21 showed no adverse effects on parturition, litter size, or growth of offspring.
8.2 Lactation
Risk Summary
Data from a published clinical lactation study reports that cephalexin is present in human milk. The Relative Infant Dose (RID) is considered to be <1% of the maternal weight adjusted dose. There are no data on the effects of cephalexin on the breastfed child or on milk production.
The development of health benefits of breastfeeding should be considered along with the mother’s clinical need for cephalexin and any potential adverse effects on the breastfed child from cephalexin or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of cephalexin in pediatric patients was established in clinical trials for the dosages described in the dosage and administration section [see Dosage and Administration (2.2 )]
8.5 Geriatric Use
Of the 701 subjects in 3 published clinical studies of cephalexin, 433 (62%) were 65 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients.
This drug is substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection [see Warnings and Precautions (5.4 )]
8.6 Renal Impairment
Cephalexin should be administered with careful monitoring in the presence of renal impairment (creatinine clearance < 30 mL/min, with or without dialysis). Under such conditions, careful clinical observation and laboratory studies renal function monitoring should be conducted because safe dosage may be lower than that usually recommended [see Dosage and Administration (2.3)].Monitor patients longer for toxicity and drug interactions due to delayed clearance.
-
10. Overdosage
Symptoms of oral overdose may include nausea, vomiting, epigastric distress, diarrhea, and hematuria. In the event of an overdose, institute general supportive measures.
Forced diuresis, peritoneal dialysis, hemodialysis, or charcoal hemoperfusion have not been established as beneficial for an overdose of cephalexin.
-
11. Description
Cephalexin capsules, USP is a semisynthetic cephalosporin antibacterial drug intended for oral administration. It is 7-(D-α-Amino-α-phenylacetamido)-3-methyl-3-cephem-4-carboxylic acid monohydrate. Cephalexin has the molecular formula C 16 H 17 N3O4SH2O and the molecular weight is 365.41.
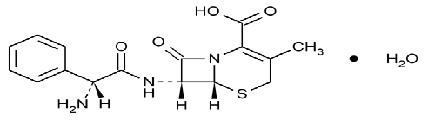
Cephalexin has the following structural formula:Each capsule contains cephalexin monohydrate equivalent to 250 mg, 333 mg, 500 mg, or 750 mg of cephalexin. The 250 mg, 333 mg, 500 mg and 750 mg capsules contain anhydrous lactose, colloidal silicon dioxide, magnesium stearate, FD & C Blue No. 1, D & C Yellow No. 10, gelatin, sodium lauryl sulphate, titanium dioxide. In addition, the 250 mg capsule contains FD & C Red No. 40; 333 mg and 750 mg Capsules contains FD & C Yellow No. 6. The imprinting ink contains; shellac, propylene glycol, strong ammonia solution and potassium hydroxide. Also black Iron oxide is used in 250mg, 333mg and 500mg and titanium dioxide is used in 750mg.
- 12. Clinical Pharmacology
-
13. Non Clinical Toxicology
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
Lifetime studies in animals have not been performed to evaluate the carcinogenic potential of cephalexin. Tests to determine the mutagenic potential of cephalexin have not been performed. In male and female rats, fertility and reproductive performance were not affected by cephalexin oral doses up to 1.5 times the highest recommended human dose based upon body surface area.
-
16. How Supplied/Storage and Handling
The 500 mg capsules are a white to off white powder filled into size 0 capsules (light green cap and light green body) that are imprinted with “219” on the both cap and body in edible black ink. They are available as follows:
Bottles of 20 Capsules: NDC: 80425-0121-04
Bottles of 28 Capsules: NDC: 80425-0121-01
Bottles of 30 Capsules: NDC: 80425-0121-03
Bottles of 40 Capsules: NDC: 80425-0121-02
Bottles of 14 Capsules: NDC: 80425-0121-05
Bottles of 5 Capsules: NDC: 80425-0121-06
Bottles of 6 Capsules: NDC: 80425-0121-07
Store at 20°C to 25°C (68°F to77°F); excursions permitted to 15 to 30°C (59 to 86°F) [see USP Controlled Room Temperature].
-
17. Patient Counseling Information
Allergic Reactions
Advise patients that allergic reactions, including serious allergic reactions, could occur and that serious reactions require immediate treatment. Ask the patient about any previous hypersensitivity reactions to cephalexin, other beta-lactams (including cephalosporins) or other allergens (5.1)Diarrhea
Advise patients that diarrhea is a common problem caused by antibacterial drugs and usually resolves when the drug is discontinued. Sometimes, frequent watery or bloody diarrhea may occur and may be a sign of a more serious intestinal infection. If severe watery or bloody diarrhea develops, advise patients to contact their healthcare providerAntibacterial Resistance
Counsel patients that antibacterial drugs including cephalexin, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cephalexin is prescribed to treat a bacterial infection, tell patients that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cephalexin or other antibacterial drugs in the future.Manufactured by:
Alkem Laboratories ltd.
Mumbai - 400 013, IndiaDistributed by:
Ascend Laboratories, LLC
Parsippany, NJ 07054Revised: January, 2019
PT 1199-10
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CEPHALEXIN
cephalexin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 80425-0121(NDC:67877-219) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEPHALEXIN (UNII: OBN7UDS42Y) (CEPHALEXIN ANHYDROUS - UNII:5SFF1W6677) CEPHALEXIN ANHYDROUS 500 mg Product Characteristics Color green Score no score Shape CAPSULE Size 22mm Flavor Imprint Code 219 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 80425-0121-4 20 in 1 BOTTLE; Type 0: Not a Combination Product 01/19/2011 2 NDC: 80425-0121-1 28 in 1 BOTTLE; Type 0: Not a Combination Product 01/19/2011 3 NDC: 80425-0121-2 40 in 1 BOTTLE; Type 0: Not a Combination Product 01/19/2011 4 NDC: 80425-0121-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/19/2011 5 NDC: 80425-0121-5 14 in 1 BOTTLE; Type 0: Not a Combination Product 01/19/2011 6 NDC: 80425-0121-6 5 in 1 BOTTLE; Type 0: Not a Combination Product 09/15/2023 7 NDC: 80425-0121-7 6 in 1 BOTTLE; Type 0: Not a Combination Product 01/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090836 01/19/2011 Labeler - Advanced Rx Pharmacy of Tennessee, LLC (117023142) Establishment Name Address ID/FEI Business Operations Advanced Rx Pharmacy of Tennessee, LLC 117023142 repack(80425-0121)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.






