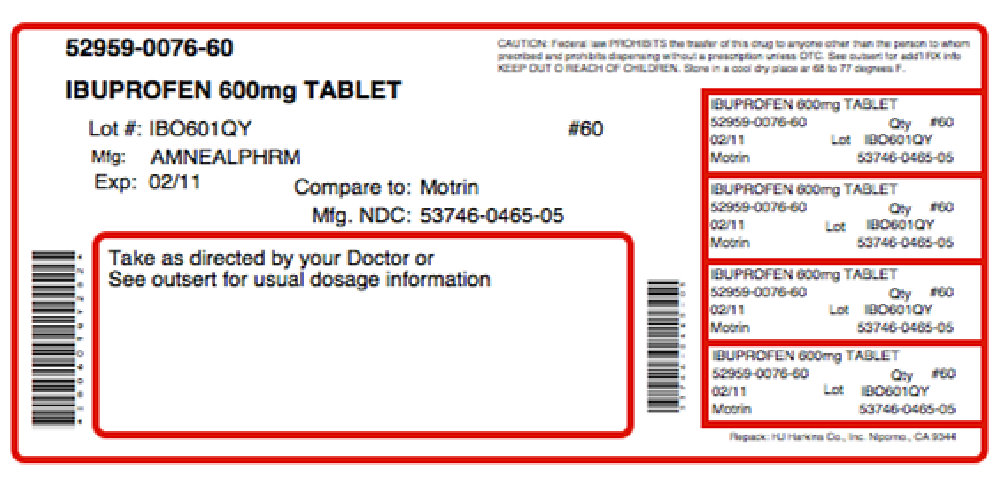
THERAPROFEN-60- ibuprofen, .gamma.-aminobutyric acid kit
Theraprofen-60 by
Drug Labeling and Warnings
Theraprofen-60 by is a Prescription medication manufactured, distributed, or labeled by Physician Therapeutics LLC, Amneal Pharmaceuticals, Targeted Medical Pharma Inc., H.J. Harkins Company, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
Cardiovascular Risk
NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk (See WARNINGS).
Ibuprofen tablets are contraindicated for treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS).
Gastrointestinal Risk
NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events. (See WARNINGS). -
DESCRIPTION
DESCRIPTION
Ibuprofen Tablets, USP contain the active ingredient ibuprofen, which is (±) – 2 – (p – isobutylphenyl) propionic acid. Ibuprofen is a white powder with a melting point of 74-77° C and is very slightly soluble in water (less than 1 mg/mL) and readily soluble in organic solvents such as ethanol and acetone.
The structural formula is represented below:
Ibuprofen Tablets, USP, a nonsteroidal anti-inflammatory drug (NSAID), is available in 400 mg, 600 mg, and 800 mg tablets for oral administration. Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, pregelatinized starch, talc, stearic acid, and titanium dioxide.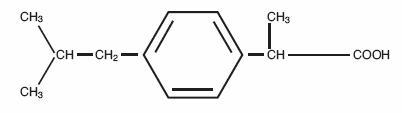
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
Ibuprofen tablets contain ibuprofen which possesses analgesic and antipyretic activities. Its mode of action, like that of other NSAIDs, is not completely understood, but may be related to prostaglandin synthetase inhibition.
In clinical studies in patients with rheumatoid arthritis and osteoarthritis, ibuprofen tablets have been shown to be comparable to aspirin in controlling pain and inflammation and to be associated with a statistically significant reduction in the milder gastrointestinal side effects (see ADVERSE REACTIONS).
Ibuprofen tablets may be well tolerated in some patients who have had gastrointestinal side effects with aspirin, but these patients when treated with ibuprofen tablets should be carefully followed for signs and symptoms of gastrointestinal ulceration and bleeding. Although it is not definitely known whether ibuprofen tablets causes less peptic ulceration than aspirin, in one study involving 885 patients with rheumatoid arthritis treated for up to one year, there were no reports of gastric ulceration with ibuprofen tablets whereas frank ulceration was reported in 13 patients in the aspirin group (statistically significant pless than .001).
Gastroscopic studies at varying doses show an increased tendency toward gastric irritation at higher doses. However, at comparable doses, gastric irritation is approximately half that seen with aspirin. Studies using 51Cr-tagged red cells indicate that fecal blood loss associated with ibuprofen tablets in doses up to 2400 mg daily did not exceed the normal range, and was significantly less than that seen in aspirin-treated patients.
In clinical studies in patients with rheumatoid arthritis, ibuprofen tablets have been shown to be comparable to indomethacin in controlling the signs and symptoms of disease activity and to be associated with a statistically significant reduction of the milder gastrointestinal (see ADVERSE REACTIONS) and CNS side effects.
Ibuprofen tablets may be used in combination with gold salts and/or corticosteroids.
Controlled studies have demonstrated that ibuprofen tablets are a more effective analgesic than propoxyphene for the relief of episiotomy pain, pain following dental extraction procedures, and for the relief of the symptoms of primary dysmenorrhea.
In patients with primary dysmenorrhea, ibuprofen tablets have been shown to reduce elevated levels of prostaglandin activity in the menstrual fluid and to reduce resting and active intrauterine pressure, as well as the frequency of uterine contractions. The probable mechanism of action is to inhibit prostaglandin synthesis rather than simply to provide analgesia.
The ibuprofen in ibuprofen tablets is rapidly absorbed. Peak serum ibuprofen levels are generally attained one to two hours after administration. With single doses up to 800 mg, a linear relationship exists between amount of drug administered and the integrated area under the serum drug concentration vs time curve. Above 800 mg, however, the area under the curve increases less than proportional to increases in dose. There is no evidence of drug accumulation or enzyme induction.
The administration of ibuprofen tablets either under fasting conditions or immediately before meals yields quite similar serum ibuprofen concentration-time profiles. When ibuprofen tablets are administered immediately after a meal, there is a reduction in the rate of absorption but no appreciable decrease in the extent of absorption. The bioavailability of the drug is minimally altered by the presence of food.
A bioavailability study has shown that there was no interference with the absorption of ibuprofen when ibuprofen tablets were given in conjunction with an antacid containing both aluminum hydroxide and magnesium hydroxide.
Ibuprofen is rapidly metabolized and eliminated in the urine. The excretion of ibuprofen is virtually complete 24 hours after the last dose. The serum half-life is 1.8 to 2.0 hours.
Studies have shown that following ingestion of the drug, 45% to 79% of the dose was recovered in the urine within 24 hours as metabolite A (25%), (+)-2-[p-(2hydroxymethyl-propyl) phenyl] propionic acid and metabolite B (37%), (+)-2-[p-(2carboxypropyl)phenyl] propionic acid; the percentages of free and conjugated ibuprofen were approximately 1% and 14%, respectively. -
INDICATIONS & USAGE
INDICATIONS AND USAGE
Carefully consider the potential benefits and risks of Ibuprofen Tablets, USP and other treatment options before deciding to use Ibuprofen Tablets, USP. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS).
Ibuprofen Tablets, USP are indicated for relief of the signs and symptoms of rheumatoid arthritis and osteoarthritis.
Ibuprofen Tablets, USP are indicated for relief of mild to moderate pain.
Ibuprofen Tablets, USP are also indicated for the treatment of primary dysmenorrhea.
Controlled clinical trials to establish the safety and effectiveness of Ibuprofen Tablets, USP in children have not been conducted. -
CONTRAINDICATIONS
CONTRAINDICATIONS
Ibuprofen tablets are contraindicated in patients with known hypersensitivity to ibuprofen.
Ibuprofen tablets should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs.
Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients (see WARNINGS, Anaphylactoid Reactions, and PRECAUTIONS, Preexisting Asthma).
Ibuprofen tablets are contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS). -
WARNINGS
WARNINGS
Pregnancy
CARDIOVASCULAR EFFECTS
Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. All NSAIDs, both COX-2 selective and nonselective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID does increase the risk of serious GI events (see GI WARNINGS).
Two large, controlled clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10-14 days following CABG surgery found an increased incidence of myocardial infarction and stroke (see CONTRAINDICATIONS).
Hypertension
NSAIDs including ibuprofen tablets, can lead to onset of new hypertension or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including ibuprofen tablets, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.
Congestive Heart Failure and Edema
Fluid retention and edema have been observed in some patients taking NSAIDs. Ibuprofen tablets should be used with caution in patients with fluid retention or heart failure.
Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation
NSAIDs, including ibuprofen tablets, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy, is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3-6 months, and in about 2-4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk.
NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients treated with neither of these risk factors. Other factors that increase the risk of GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population. To minimize the potential risk for an adverse GI event in patients treated with a NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulcerations and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high-risk patients, alternate therapies that do not involve NSAIDs should be considered.
Renal Effects
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a NSAID may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
Advanced Renal Disease
No information is available from controlled clinical studies regarding the use of ibuprofen tablets in patients with advanced renal disease. Therefore, treatment with ibuprofen tablets is not recommended in these patients with advanced renal disease. If ibuprofen tablets therapy must be initiated, close monitoring of the patients renal function is advisable.
Anaphylactoid Reactions
As with other NSAIDs, anaphylactoid reactions may occur in patients without known prior exposure to ibuprofen tablets. Ibuprofen tablets should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs (see CONTRAINDICATIONS and PRECAUTIONS, Preexisting Asthma). Emergency help should be sought in cases where an anaphylactoid reaction occurs.
Skin Reactions
NSAIDs, including ibuprofen tablets, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
In late pregnancy, as with other NSAIDs, ibuprofen tablets should be avoided because it may cause premature closure of the ductus arteriosus. -
PRECAUTIONS
PRECAUTIONS
General
Ibuprofen tablets cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.
The pharmacological activity of ibuprofen tablets in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.
Hepatic effects
Borderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs, including ibuprofen tablets. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continuing therapy. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice, fulminant hepatitis, liver necrosis, and hepatic failure, some of them with fatal outcomes have been reported.
A patient with symptoms and/or signs suggesting liver dysfunction, or with abnormal liver test values, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with ibuprofen tablets. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), ibuprofen tablets should be discontinued.
Hematological effects
Anemia is sometimes seen in patients receiving NSAIDs, including ibuprofen tablets. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including ibuprofen tablets, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia.
In two postmarketing clinical studies the incidence of a decreased hemoglobin level was greater than previously reported. Decrease in hemoglobin of 1 gram or more was observed in 17.1% of 193 patients on 1600 mg ibuprofen daily (osteoarthritis), and in 22.8% of 189 patients taking 2400 mg of ibuprofen daily (rheumatoid arthritis). Positive stool occult blood tests and elevated serum creatinine levels were also observed in these studies.
NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients.
Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible.
Patients receiving ibuprofen tablets who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants should be carefully monitored.
Preexisting asthma
Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm, which can be fatal. Since cross reactivity, including bronchospasm, between aspirin and NSAIDs has been reported in such aspirin-sensitive patients, ibuprofen tablets should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with preexisting asthma.
Ophthalmological effects
Blurred and/or diminished vision, scotomata, and/or changes in color vision have been reported. If a patient develops such complaints while receiving ibuprofen tablets, the drug should be discontinued, and the patient should have an ophthalmologic examination which includes central visual fields and color vision testing.
Aseptic meningitis
Aseptic meningitis with fever and coma has been observed on rare occasions in patients on ibuprofen therapy. Although it is probably more likely to occur in patients with systemic lupus erythematosus and related connective tissue diseases, it has been reported in patients who do not have an underlying chronic disease. If signs or symptoms of meningitis develop in a patient on ibuprofen tablets, the possibility of its being related to ibuprofen tablets should be considered. -
INFORMATION FOR PATIENTS
INFORMATION FOR PATIENTS
Patients should be informed of the following information before initiating therapy with an NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.
Ibuprofen tablets, like other NSAIDs, may cause serious CV side effects, such as MI or stroke, which may result in hospitalization and even death. Although serious CV events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and should ask for medical advice when observing any indicative signs or symptoms. Patients should be apprised of the importance of this follow-up (see WARNINGS, Cardiovascular Effects).
Ibuprofen tablets, like other NSAIDs, can cause GI discomfort and, rarely, serious GI side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Although serious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing any indicative signs or symptoms including epigastric pain, dyspepsia, melena, and hematemesis. Patients should be apprised of the importance of this follow-up (see WARNINGS, Gastrointestinal Effects-Risk of Ulceration, Bleeding and Perforation).
Ibuprofen tablets, like other NSAIDs, can cause serious skin side effects such as exfoliative dermatitis, SJS and TEN, which may result in hospitalization and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching, and should ask for medical advice when observing any indicative signs or symptoms. Patients should be advised to stop the drug immediately if they develop any type of rash and contact their physicians as soon as possible.
Patients should promptly report signs or symptoms of unexplained weight gain or edema to their physicians.
Patients should be informed of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness and "flu-like" symptoms).If these occur, patients should be instructed to stop therapy and seek immediate medical therapy.
Patients should be informed of the signs of an anaphylactoid reaction (e.g., difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help (see WARNINGS).
In late pregnancy, as with other NSAIDs, ibuprofen tablets should be avoided because it may cause premature closure of the ductus arteriosus. -
LABORATORY TESTS
Laboratory Tests
Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding.
Patients on long-term treatment with NSAIDs should have their CBC and chemistry profile checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, rash etc.), or abnormal liver tests persist or worsen, Ibuprofen Tablets, USP should be discontinued. -
DRUG INTERACTIONS
Drug Interactions
ACE-inhibitors:
Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE-inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE-inhibitors.
Aspirin
When ibuprofen tablets are administered with aspirin, its protein binding is reduced, although the clearance of free ibuprofen tablets is not altered.
The clinical significance of this interaction is not known; however, as with other NSAIDs, concomitant administration of ibuprofen and aspirin is not generally recommended because of the potential for increased adverse effects.
Diuretics
Clinical studies, as well as post marketing observations, have shown that ibuprofen tablets can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure (see WARNINGS, Renal Effects), as well as to assure diuretic efficacy.
Lithium
Ibuprofen produced an elevation of plasma lithium levels and a reduction in renal lithium clearance in a study of eleven normal volunteers. The mean minimum lithium concentration increased 15% and the renal clearance of lithium was decreased by 19% during this period of concomitant drug administration.
This effect has been attributed to inhibition of renal prostaglandin synthesis by ibuprofen. Thus, when ibuprofen and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity. (Read circulars for lithium preparation before use of such concurrent therapy.)
Methotrexate
NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.
Warfarin-type anticoagulants
Several short-term controlled studies failed to show that ibuprofen tablets significantly affected prothrombin times or a variety of other clotting factors when administered to individuals on coumarin-type anticoagulants. However, because bleeding has been reported when ibuprofen tablets and other NSAIDs have been administered to patients on coumarin-type anticoagulants, the physician should be cautious when administering ibuprofen tablets to patients on anticoagulants. The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that the users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone.
H-2 Antagonists
In studies with human volunteers, co-administration of cimetidine or ranitidine with ibuprofen had no substantive effect on ibuprofen serum concentrations. -
PREGNANCY
Pregnancy
Teratogenic effects: Pregnancy Category C
Reproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities. However, animal reproduction studies are not always predictive of human response.
There are no adequate and well-controlled studies in pregnant women. Ibuprofen tablets should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus. - NONTERATOGENIC EFFECTS
- LABOR & DELIVERY
-
NURSING MOTHERS
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from ibuprofen tablets, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother. - PEDIATRIC USE
- GERIATRIC USE
-
ADVERSE REACTIONS
ADVERSE REACTIONS
The most frequent type of adverse reaction occurring with ibuprofen tablets is gastrointestinal. In controlled clinical trials the percentage of patients reporting one or more gastrointestinal complaints ranged from 4% to 16%.
In controlled studies when ibuprofen tablets were compared to aspirin and indomethacin in equally effective doses, the overall incidence of gastrointestinal complaints was about half that seen in either the aspirin- or indomethacin-treated patients.
Adverse reactions observed during controlled clinical trials at an incidence greater than 1% are listed in the table. Those reactions listed in Column one encompass observations in approximately 3,000 patients. More than 500 of these patients were treated for periods of at least 54 weeks. Still other reactions occurring less frequently than 1 in 100 were reported in controlled clinical trials and from marketing experience. These reactions have been divided into two categories: Column two of the table lists reactions with therapy with ibuprofen tablets where the probability of a causal relationship exists: for the reactions in Column three, a causal relationship with ibuprofen tablets has not been established.
Reported side effects were higher at doses of 3200 mg/day than at doses of 2400 mg or less per day in clinical trials of patients with rheumatoid arthritis. The increases in incidence were slight and still within the ranges reported in the table.
to report suspected adverse reactions and 1-800-FDA-1088
* Reactions occurring in 3% to 9% of patients treated with ibuprofen tablets. (Those reactions occurring in less than 3% of the patients are unmarked.)Incidence Greater Than 1%
(but less than 3%)
Probable Causal RelationshipPrecise Incidence Unknown
(but less than 1%)
Probable Causal Relationship**Precise Incidence Unknown
(but less than 1%)
Causal Relationship Unknown**GASTROINTESTINAL
Nausea*, epigastric pain*, heartburn*, diarrhea, abdominal distress, nausea and vomiting, indigestion, constipation, abdominal cramps or Pain, fullness of GI tract (bloating and flatulence)
Gastric or duodenal ulcer with bleeding and/or perforation, gastrointestinal hemorrhage, melena, gastritis, hepatitis, jaundice, abnormal liver function tests; pancreatitis
CENTRAL NERVOUS SYSTEM
Dizziness*, headache, nervousness
Depression, insomnia, confusion, emotional liability, somnolence, aseptic meningitis with fever and coma (see PRECAUTIONS)
Paresthesias, hallucinations, dream abnormalities, pseudo-tumor cerebri
DERMATOLOGIC
Rash* (including maculopapular type), pruritus
Vesiculobullous eruptions, urticaria, erythema multiforme, Stevens-Johnson syndrome, alopecia
Toxic epidermal necrolysis, photoallergic skin reactions neuritis, cataracts
SPECIAL SENSES
Tinnitus
Hearing loss, amblyopia (blurred and/or diminished vision, scotomata and/or changes in color vision) (see PRECAUTIONS)
Conjunctivitis, diplopia, optic neuritis, cataracts
HEMATOLOGIC
Neutropenia, agranulocytosis, aplastic anemia, hemolytic anemia (sometimes Coombs positive), thrombocytopenia with or without purpura, eosinophilia, decreases in hemoglobin and hematocrit (see PRECAUTIONS)
Bleeding episodes (eg epistaxis, menorrhagia)
METABOLIC/ENDOCRINE
Decreased appetite
Gynecomastia, hypoglycemic reaction, acidosis
CARDIOVASCULAR
Edema, fluid retention (generally responds promptly to drug discontinuation) (see PRECAUTIONS)
Congestive heart failure in patients with marginal cardiac function, elevated blood pressure, palpitations
Arrhythmias (sinus tachycardia, sinus bradycardia)
ALLERGIC
Syndrome of abdominal pain, fever, chills, nausea and vomiting; anaphylaxis; bronchospasm (see CONTRAINDICATIONS)
Serum sickness, lupus erythe- matosus syndrome. Henoch-Schonlein vasculitis, angioedema
RENAL
Acute renal failure (see PRECAUTIONS), decreased creatinine clearance, polyuria, azotemia, cystitis, Hematuria
Renal papillary necrosis
MISCELLANEOUS
Dry eyes and mouth, gingival ulcer, rhinitis
** Reactions are classified under “Probable Causal Relationship (PCR)” if there has been one positive rechallenge or if three or more cases occur which might be causally related. Reactions are classified under “Causal Relationship Unknown” if seven or more events have been reported but the criteria for PCR have not been met.
-
OVERDOSAGE
OVERDOSAGE
Approximately 1 ½ hours after the reported ingestion of from 7 to 10 ibuprofen tablets (400 mg), a 19-month old child weighing 12 kg was seen in the hospital emergency room, apneic and cyanotic, responding only to painful stimuli. This type of stimulus, however, was sufficient to induce respiration. Oxygen and parenteral fluids were given; a greenish-yellow fluid was aspirated from the stomach with no evidence to indicate the presence of ibuprofen. Two hours after ingestion the child's condition seemed stable; she still responded only to painful stimuli and continued to have periods of apnea lasting from 5 to 10 seconds. She was admitted to intensive care and sodium bicarbonate was administered as well as infusions of dextrose and normal saline. By four hours post-ingestion she could be aroused easily, sit by herself and respond to spoken commands. Blood level of ibuprofen was 102.9 μg/mL approximately 8 ½ hours after accidental ingestion. At 12 hours she appeared to be completely recovered.
In two other reported cases where children (each weighing approximately 10 kg) accidentally, acutely ingested approximately 120 mg/kg, there were no signs of acute intoxication or late sequelae. Blood level in one child 90 minutes after ingestion was 700 μg/mL - about 10 times the peak levels seen in absorption-excretion studies.
A 19-year old male who had taken 8,000 mg of ibuprofen over a period of a few hours complained of dizziness, and nystagmus was noted. After hospitalization, parenteral hydration and three days bed rest, he recovered with no reported sequelae.
In cases of acute overdosage, the stomach should be emptied by vomiting or lavage, though little drug will likely be recovered if more than an hour has elapsed since ingestion. Because the drug is acidic and is excreted in the urine, it is theoretically beneficial to administer alkali and induce diuresis. In addition to supportive measures, the use of oral activated charcoal may help to reduce the absorption and reabsorption of ibuprofen tablets. -
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION
Carefully consider the potential benefits and risks of ibuprofen tablets and other treatment options before deciding to use ibuprofen tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS).
After observing the response to initial therapy with ibuprofen tablets the dose and frequency should be adjusted to suit an individual patient's needs.
Do not exceed 3200 mg total daily dose. If gastrointestinal complaints occur, administer Ibuprofen Tablets, USP with meals or milk.
Rheumatoid arthritis and osteoarthritis, including flare-ups of chronic disease:
Suggested Dosage: 1200 mg-3200 mg daily (300 mg qid; 400 mg, 600 mg or 800 mg tid or qid).
Individual patients may show a better response to 3200 mg daily, as compared with 2400 mg, although in well-controlled clinical trials patients on 3200 mg did not show a better mean response in terms of efficacy. Therefore, when treating patients with 3200 mg/day, the physician should observe sufficient increased clinical benefits to offset potential increased risk.
The dose should be tailored to each patient, and may be lowered or raised depending on the severity of symptoms either at time of initiating drug therapy or as the patient responds or fails to respond.
In general, patients with rheumatoid arthritis seem to require higher doses of ibuprofen tablets than do patients with osteoarthritis.
The smallest dose of ibuprofen tablets that yields acceptable control should be employed. A linear blood level dose-response relationship exists with single doses up to 800 mg (See CLINICAL PHARMACOLOGY for effects of food on rate of absorption). The availability of four tablet strengths facilitates dosage adjustment.
In chronic conditions, a therapeutic response to therapy with ibuprofen tablets is sometimes seen in a few days to a week but most often is observed by two weeks. After a satisfactory response has been achieved, the patient's dose should be reviewed and adjusted as required.
Mild to moderate pain: 400 mg every 4 to 6 hours as necessary for relief of pain.
In controlled analgesic clinical trials, doses of ibuprofen tablets greater than 400 mg were no more effective than the 400 mg dose.
Dysmenorrhea: For the treatment of dysmenorrhea, beginning with the earliest onset of such pain, ibuprofen tablets should be given in a dose of 400 mg every 4 hours as necessary for the relief of pain. -
HOW SUPPLIED
HOW SUPPLIED
Ibuprofen Tablets, USP are available in the following strengths, colors and sizes:
400 mg (white, round, biconvex, aqueous film-coated tablets, debossed “IP 464” on obverse and plain on reverse.
They are available as follows:
Bottles of 30: NDC: 53746-464-30
Bottles of 60: NDC: 53746-464-60
Bottles of 90: NDC: 53746-464-90
Bottles of 100: NDC: 53746-464-01
Bottles of 500: NDC: 53746-464-05
600 mg (white, oval-shaped, biconvex, aqueous film-coated tablets, debossed “IP 465” on obverse and plain on reverse.
They are available as follows:
Bottles of 30: NDC: 53746-465-30
Bottles of 50: NDC: 53746-465-50
Bottles of 60: NDC: 53746-465-60
Bottles of 90: NDC: 53746-465-90
Bottles of 100: NDC: 53746-465-01
Bottles of 500: NDC: 53746-465-05
800 mg (white, capsule-shaped, biconvex, aqueous film-coated tablets, debossed “IP 466” on obverse and plain on reverse.
They are available as follows:
Bottles of 30: NDC: 53746-466-30
Bottles of 50: NDC: 53746-466-50
Bottles of 60: NDC: 53746-466-60
Bottles of 90: NDC: 53746-466-90
Bottles of 100: NDC: 53746-466-01
Bottles of 500: NDC: 53746-466-05Rx only
Manufactured by:
Amneal Pharmaceuticals of NY
Hauppauge, NY 11788
Distributed by:
Amneal Pharmaceuticals
Glasgow, KY 42141
Rev. 09-2009 - STORAGE AND HANDLING
-
PRINCIPAL DISPLAY PANEL
NDC: 53746-466-01 Ibuprofen Tablets, USP 600 mg PHARMACIST: Dispense with Medication Guide provided separately to each patient. IP 465 Rx only 100 TABLETS amneal PHARMACEUTICALS Each film-coated tablet contains: Ibuprofen, USP..............600 mg See package insert for complete product information. Dispense in tight container. Store at controlled room temperature 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Manufactured by: Amneal Pharmaceuticals of NY Hauppauge, NY 11788 Distributed by: Amneal Pharmaceuticals Glasgow, KY 42141 Rev. 07-2008 N3 53746-465-01 6 Lot No: 3/4' Exp. Date: Non-Varnish Area
-
SPL UNCLASSIFIED SECTION
Theramine (U.S. patent pending) capsules by oral administration. A specially formulated Medical Food product, consisting of a proprietary blend of amino acids and polyphenol ingredients in specific proportions, for the dietary management of the metabolic processes associated with pain disorders and inflammatory conditions. (PD) (IC). Must be administered under physician supervision.
Medical Foods
Medical Food products are often used in hospitals (e.g., for burn victims or kidney dialysis patients) and outside of a hospital setting under a physician’s care for the dietary management of diseases in patients with particular, unique or distinctive medical or metabolic needs due to their disease or condition. Congress defined "Medical Food" in the Orphan Drug Act and Amendments of 1988 as "a food which is formulated to be consumed or administered enterally [or orally] under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation." Medical Foods are complex formulated products, requiring sophisticated and exacting technology, and that are used only for a patient receiving active and ongoing medical supervision wherein the patient requires medical care on a recurring basis for, among other things, instructions on the use of the Medical Food. Theramine has been developed, manufactured, and labeled in accordance with both the statutory definition of a Medical Food and FDA’s regulatory labeling guidelines. Theramine must be used while the patient is under the ongoing care of a physician.
PAIN DISORDERS (PD) INFLAMMATORY CONDITIONS (IC)
PD and IC as a Metabolic Deficiency Disease
A critical component of the definition of a Medical Food is that the product must address the distinct nutritional requirements of a particular disease or condition. FDA scientists have proposed a physiologic definition of distinctive nutritional requirements as follows: “the dietary management of patients with specific diseases requires, in some instances, the ability to meet nutritional requirements that differ substantially from the needs of healthy persons. For example, in establishing the recommended dietary allowances for general, healthy population, the Food and Nutrition Board of the Institute of Medicine National Academy of Sciences, recognized that different or distinctive physiologic requirements may exist for certain persons with "special nutritional needs arising from metabolic disorders, chronic diseases, injuries, premature birth, other medical conditions and drug therapies. Thus, the distinctive nutritional needs associated with a disease reflects the total amount needed by a healthy person to support life or maintain homeostasis, adjusted for the distinctive changes in the nutritional needs of the patient as a result of the effects of the disease process on absorption, metabolism and excretion.” It was also proposed that in patients with certain disease states who respond to nutritional therapies, a physiologic deficiency of the nutrient is assumed to exist. For example, if a patient with a pain disorder responds to a tryptophan formulation by decreasing perceived pain, then a deficiency of tryptophan is assumed to exist. Patients with pain disorders and inflammatory conditions are known to have increased nutritional requirements for tryptophan, choline, arginine, GABA, flavonoids, and certain antioxidants. These nutritional requirements are such that they cannot be achieved by the modification of the normal diet alone, or by supplementing the diet.
Patients with pain disorders and inflammatory conditions frequently exhibit reduced plasma levels of tryptophan and GABA, and have been shown to respond to oral administration of GABA, arginine, tryptophan, or a 5-hydroxytryptophan formulation. Research has shown that tryptophan, arginine or GABA reduced diets result in a fall of circulating tryptophan, arginine, and/or GABA. Patients with pain disorders frequently exhibit activation of the degradation pathways that increases the turnover rate of GABA, arginine and/or tryptophan leading to a reduced level of production of serotonin, GABA or nitric oxide for a given precursor blood level. Research has also shown that a genetic predisposition to accelerated degradation can lead to increased precursor requirements in certain patients with pain disorders and inflammatory conditions.
Choline is required to fully potentiate acetylcholine synthesis by brain neurons. A deficiency of choline leads to reduced acetylcholine production by the neurons. Provision of tryptophan, arginine, GABA, choline and flavonoids with antioxidants, in specific proportions can restore the production of beneficial serotonin, nitric oxide, and acetylcholine, thereby reducing the perception of pain and reducing inflammation. L-Histidine is known to produce brain histamine that stimulates production of ACTH, producing cortisol to reduce inflammation.
-
DESCRIPTION
PRODUCT DESCRIPTION
Primary Ingredients
Theramine consists of a proprietary formulation of Gamma Aminobutyric Acid, Choline Bitartrate, Whey Protein Hydrolysate, L-Arginine, L-Histidine, L-Glutamine, Theobromine, Griffonia See, Grape Seed, L-Serine, and Cinnamon in specific proportions. These ingredients fall into the classification of Generally Recognized as Safe (GRAS) as defined by the Food and Drug Administration (FDA) (Sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act). A GRAS substance is distinguished from a food additive on the basis of the common knowledge about the safety of the substance for its intended use. The standard for an ingredient to achieve GRAS status requires not only technical demonstration of non-toxicity and safety, but also general recognition of safety through widespread usage and agreement of that safety by experts in the field. Many ingredients have been determined by the FDA to be GRAS, and are listed as such by regulation, in Volume 21 Code of Federal Regulations (CFR) Sections 182, 184, and 186.
Amino Acids
Amino Acids are the building blocks of protein and are GRAS listed as they have been safely ingested by humans for thousands of years. The formulations of the amino acids in Theramine are equivalent to those found in the usual human diet. Patients with pain disorders may require an increased amount of certain amino acids that cannot be obtained from normal diet alone. Tryptophan, for example, is an obligatory amino acid. The body cannot make tryptophan and must obtain tryptophan from the diet. Tryptophan is needed to produce serotonin. Serotonin is required to reduce pain. Patients with pain disorders and inflammatory conditions have altered serotonin metabolism.
Flavonoids
Flavonoids are a group of phytochemical compounds found in all vascular plants including fruits and vegetables. They are a part of a larger class of compounds known as polyphenols. Many of the therapeutic or health benefits of colored fruits and vegetables, cocoa, red wine, and green tea are directly related to their flavonoid content. The specially formulated flavonoids found in Theramine cannot be obtained from conventional foods in the necessary proportions to elicit a therapeutic response.
Other Ingredients
Theramine contains the following “inactive” or other ingredients, as fillers, excipients, and colorings: Gelatin, Silicon Dioxide, Tricalcium Phosphate, Vegetable Magnesium stearate, Cellulose, FDandC Blue#1, FDandC Red #3, Titanium Dioxide.
Physical Description
Theramine is a yellow to light brown powder. Theramine contains Gamma Aminobutyric Acid, Choline Bitartrate, Whey Protein Hydrolysate, L-Arginine, L-Histidine HCL, L-Glutamine, Theobromine, Griffonia Seed, Grape Seed, L-Serine, and Cinnamon. Each capsule consists of a proprietary blend of these ingredients in an amount of 366mg or 732mg per two (2) capsule dose.
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
Mechanism of Action
Theramine acts by restoring and maintaining the balance of the neurotransmitters GABA, nitric oxide, serotonin, and acetylcholine that are associated with pain disorders and inflammatory conditions. Theramine stimulates the production ACTH to reduce inflammation.
Metabolism
The amino acids in Theramine are primarily absorbed by the stomach and small intestines. All cells metabolize the amino acids in Theramine. Circulating tryptophan, arginine and choline blood levels determine the production of serotonin, nitric oxide, and acetylcholine.
Excretion
Theramine is not an inhibitor of cytochrome P450 1A2, 2C9, 2C19, 2D6, or 3A4. These isoenzymes are principally responsible for 95% of all detoxification of drugs, with CYP3A4 being responsible for detoxification of roughly 50% of drugs. Amino acids do not appear to have an effect on drug metabolizing enzymes.
- INDICATIONS & USAGE
-
CLINICAL STUDIES
CLINICAL EXPERIENCE
Administration of Theramine has demonstrated significant reduction in symptoms of pain and inflammation in patients with acute and chronic pain when used
for the dietary management of the metabolic processes associated with pain disorders and inflammatory conditions. Administration of Theramine results in
the induction and maintenance of pain relief in patients with pain disorders and inflammatory conditions.
- CONTRAINDICATIONS
-
ADVERSE REACTIONS
ADVERSE REACTIONS
Ingestion of L-Tryptophan, L-Arginine, or Choline at high doses of up to 15 grams daily is generally well tolerated. The most common adverse reactions of higher
doses — from 15 to 30 grams daily — are nausea, abdominal cramps, and diarrhea. Theramine contains less than 1 gram per dose of amino acids however, some patients
may experience these symptoms at lower doses. The total combined amount of amino acids in each Theramine capsule does not exceed 300 mg. - DRUG INTERACTIONS
-
OVERDOSAGE
OVERDOSE
There is a negligible risk of overdose with Theramine as the total amount of amino acids in a one month supply (90 capsules) is less than 30 grams. Overdose symptoms may include diarrhea, weakness, and nausea.
POST-MARKETING SURVEILLANCE
Post-marketing surveillance has shown no serious adverse reactions. Reported cases of mild rash and itching may have been associated with allergies to Theramine flavonoid ingredients, including Cinnamon, Cocoa, and Grape Seed. These reactions were temporary, transient in nature and subsided within 24-hours. -
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION
Recommended Administration
For the dietary management of the metabolic processes associated with pain disorders and inflammatory conditions. Take two (2) capsules every four hours or as directed by physician. As with most amino acid formulations Theramine should be taken without food to increase the absorption of key ingredients. -
HOW SUPPLIED
How Supplied
Theramine is supplied in purple and white, size 0 capsules in bottles of 60 and 90 capsules.
Physician Supervision
Theramine is a Medical Food product available by prescription only and may be used per FDA law, and product labeling only while the patient is under ongoing physician supervision.
U.S. patent pending
Manufactured by Arizona Nutritional Supplements, Inc. Chandler AZ 85225
Distributed exclusively by Physician Therapeutics LLC, a wholly owned subsidiary of Targeted Medical Pharma Inc. Los Angeles, CA
www.ptlcentral.com NDC: 68405-008-02 NDC: 68405-008-03 - STORAGE AND HANDLING
-
PRINCIPAL DISPLAY PANEL
68405-008-02 Directions for use: Must be administered under physician supervision. For adults only. As a Medical Food, take one (1) or two (2) capsules every four hours or as directed by physician. For the dietary management of Myalgia. Contains no added sugar, starch, wheat, yeast, preservatives, artificial flavor. Storage: Keep tightly closed in a cool dry place 8-32°C (45-90°F), relative humidity, below 50%. Warning: Keep this product out of the reach of children. NDC# 68405-008-02 LOT# 007007 PHYSICIAN THERAPEUTICS THERAMINE Medical Food Rx only 60 Capsules Ingredients: Each serving (per 2 capsules) contains: Proprietary Amino Acid Formulation Whey Protein Hydrolysate (milk sourced isolate), L-Arginine (as L-Arginine HCl), L-Histidine HCI, L-Glutamine, L-Serine Gamma Amino Butyric Acid Choline Bitartrate Griffonia Seed Extract (5-HTP) Cocoa Extract (6% Theobromine) Grape Seed Extract (85% Polyphenols) Cinnamon (bark) Other Ingredients: Gelatin, Tricalcium Phosphate, Silicon Dioxide, Magnesium Stearate, Microcrystalline Cellulose, Titanium Dioxide, FDandC Red #3, FDandC Blue #1. Distributed exclusively by: Physicians Therapeutics A Division of Targeted Medical Pharma, Inc. Los Angeles, CA 90077 www.ptlcentral.com US Patent 7,582,315; 7,585,523; 7,595,067; 7,601,369. LOT 007007 EXP 08/13
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THERAPROFEN-60
ibuprofen, .gamma.-aminobutyric acid kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68405-880 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68405-880-26 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 60 Part 2 1 BOTTLE 60 Part 1 of 2 IBUPROFEN
ibuprofen tabletProduct Information Item Code (Source) NDC: 52959-076(NDC:53746-465) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 600 mg Product Characteristics Color white (WHITE) Score no score Shape OVAL (Biconvex) Size 17mm Flavor Imprint Code IP;465 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52959-076-60 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078558 05/19/2011 Part 2 of 2 THERAMINE 60
.gamma.-aminobutyric acid capsuleProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength .GAMMA.-AMINOBUTYRIC ACID (UNII: 2ACZ6IPC6I) (.GAMMA.-AMINOBUTYRIC ACID - UNII:2ACZ6IPC6I) .GAMMA.-AMINOBUTYRIC ACID 100 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRICALCIUM PHOSPHATE (UNII: K4C08XP666) MAGNESIUM STEARATE (UNII: 70097M6I30) POWDERED CELLULOSE (UNII: SMD1X3XO9M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color purple (PURPLE, WHITE) Score no score Shape CAPSULE Size 20mm Flavor Imprint Code ; Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Medical Food 05/19/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/19/2011 Labeler - Physician Therapeutics LLC (931940964) Establishment Name Address ID/FEI Business Operations Amneal Pharmaceuticals 831227801 manufacture Establishment Name Address ID/FEI Business Operations Targeted Medical Pharma Inc. 126962740 manufacture Establishment Name Address ID/FEI Business Operations H.J. Harkins Company, Inc. 147681894 repack
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.