MINERAL SUNSCREEN- zinc oxide spf 50 lotion
Mineral Sunscreen by
Drug Labeling and Warnings
Mineral Sunscreen by is a Otc medication manufactured, distributed, or labeled by ALAINA HEALTHCARE PRIVATE LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
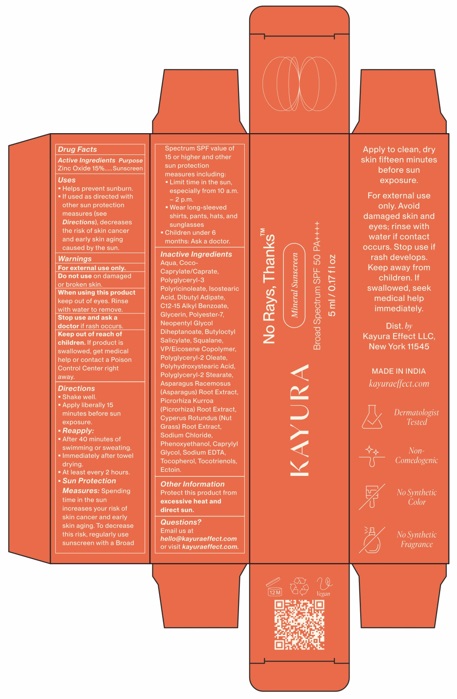
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- Shake well.
- Apply liberally 15 minutes before sun exposure.
- Reapply:
- After 40 minutes of swimming or sweating.
- Immediately after towel drying.
- At least every 2 hours.
-
Sun Protection Measures:Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses.
- Children under 6 months: ask a doctor.
-
Inactive Ingredients
Aqua, Coco-Caprylate/Caprate, Polyglyceryl-3 Polyricinoleate, Isostearic Acid, Dibutyl Adipate, C12-15 Alkyl Benzoate, Glycerin, Polyester-7, Neopentyl Glycol Diheptanoate, Butyloctyl Salicylate, Squalane, VP/Eicosene Copolymer, Polyglyceryl-2 Oleate, Polyhydroxystearic Acid, Polyglyceryl-2 Stearate, Asparagus Racemosus (Asparagus) Root Extract, Picrorhiza Kurroa (Picrorhiza) Root Extract, Cyperus Rotundas (Nut Grass) Root Extract, Sodium Chloride, Phenoxyethanol, Caprylyl Glycol, Sodium EDTA, Tocopherol, Tocotrienols, Ectoin.
- Other Information
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MINERAL SUNSCREEN
zinc oxide spf 50 lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 73492-152 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 15 g in 100 mL Inactive Ingredients Ingredient Name Strength AQUA (UNII: 059QF0KO0R) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) ISOSTEARIC ACID (UNII: X33R8U0062) DIBUTYL ADIPATE (UNII: F4K100DXP3) C12-15 ALKYL BENZOATE (UNII: A9EJ3J61HQ) GLYCERIN (UNII: PDC6A3C0OX) POLYESTER-7 (UNII: 0841698D2F) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SQUALANE (UNII: GW89575KF9) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) POLYGLYCERYL-2 OLEATE (UNII: 5759J47SAM) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYGLYCERYL-2 STEARATE (UNII: 253MC0P0YV) ASPARAGUS RACEMOSUS ROOT (UNII: Y8P1YR4920) PICRORHIZA KURROA ROOT (UNII: ZB5K2O2999) CYPERUS ROTUNDUS ROOT (UNII: 4B51SRR959) SODIUM CHLORIDE (UNII: 451W47IQ8X) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) EDETATE SODIUM (UNII: MP1J8420LU) TOCOTRIENOLS (UNII: KP2MW85SSQ) ECTOIN (UNII: 7GXZ3858RY) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73492-152-50 1 in 1 CARTON 03/07/2025 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 73492-152-05 1 in 1 CARTON 03/07/2025 2 5 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/04/2025 Labeler - ALAINA HEALTHCARE PRIVATE LIMITED (858720927) Establishment Name Address ID/FEI Business Operations ALAINA HEALTHCARE PRIVATE LIMITED 858720927 manufacture(73492-152)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.