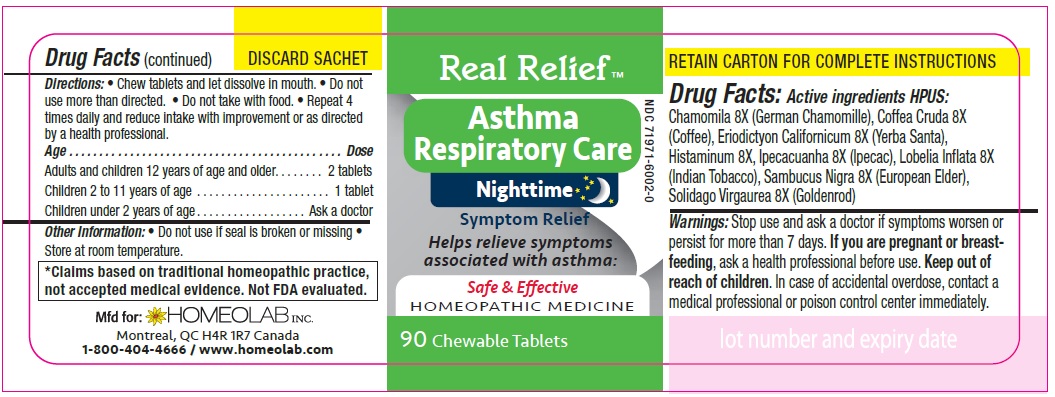
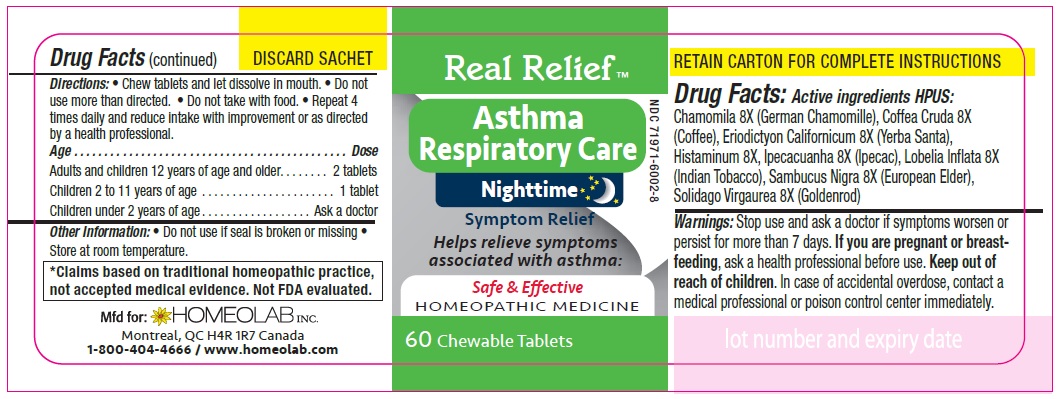
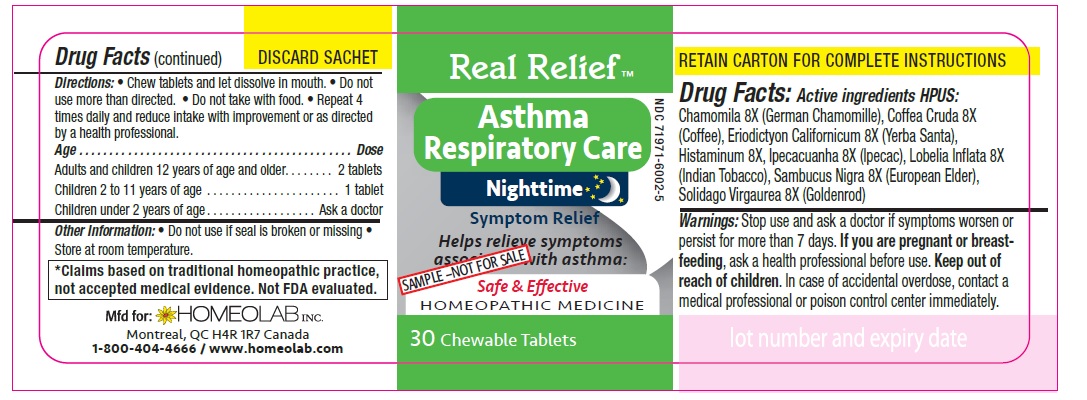
REAL RELIEF- chamomila, coffea cruda, eriodictyon californicum, histaminum, ipecacuanha, lobelia inflata, sambucus nigra, solidago virgaurea tablet
Real Relief by
Drug Labeling and Warnings
Real Relief by is a Homeopathic medication manufactured, distributed, or labeled by GMP Laboratories of America, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
-
PURPOSE
Purpose
Homeopathic remedy helps relieve symptoms of asthma:
- gasping for air
- dry cough
- difficulty breathing
- irritability and nocturnal awakenings
- wheezing
- cough with mucus, cough with expectoration
- restlessness and sleeplessness
- excess mucus
- irregular respiration
The letters 'HPUS' indicate that the components in this product are officially monographed in the Homoeopathic Pharmacopoeia of the United States.
*Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
Chew tablets and let dissolve in mouth.
Do not use more than directed.
Do not take with food.
Repeat 4 times daily and reduce intake with improvement or as directed by a health professional
Age…………………………………………………………Dose
Adults and Children 12 years of age and older………...... 2 tablets
Children 2 to 11 years of age…...……………………............ 1 tablet
Children under 2 years of age……………………................. Ask a doctor
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- Product label
-
INGREDIENTS AND APPEARANCE
REAL RELIEF
chamomila, coffea cruda, eriodictyon californicum, histaminum, ipecacuanha, lobelia inflata, sambucus nigra, solidago virgaurea tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 65808-322 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MATRICARIA CHAMOMILLA (UNII: G0R4UBI2ZZ) (MATRICARIA CHAMOMILLA - UNII:G0R4UBI2ZZ) MATRICARIA CHAMOMILLA 8 [hp_X] ARABICA COFFEE BEAN (UNII: 3SW678MX72) (ARABICA COFFEE BEAN - UNII:3SW678MX72) ARABICA COFFEE BEAN 8 [hp_X] ERIODICTYON CALIFORNICUM LEAF (UNII: 2Y7TIQ135H) (ERIODICTYON CALIFORNICUM LEAF - UNII:2Y7TIQ135H) ERIODICTYON CALIFORNICUM LEAF 8 [hp_X] HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 8 [hp_X] IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 8 [hp_X] LOBELIA INFLATA (UNII: 9PP1T3TC5U) (LOBELIA INFLATA - UNII:9PP1T3TC5U) LOBELIA INFLATA 8 [hp_X] SAMBUCUS NIGRA FLOWERING TOP (UNII: CT03BSA18U) (SAMBUCUS NIGRA FLOWERING TOP - UNII:CT03BSA18U) SAMBUCUS NIGRA FLOWERING TOP 8 [hp_X] SOLIDAGO VIRGAUREA FLOWERING TOP (UNII: 5405K23S50) (SOLIDAGO VIRGAUREA FLOWERING TOP - UNII:5405K23S50) SOLIDAGO VIRGAUREA FLOWERING TOP 8 [hp_X] Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape ROUND Size 9mm Flavor Imprint Code HLB Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65808-322-01 1 in 1 CARTON 01/01/2020 1 90 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/01/2020 Labeler - GMP Laboratories of America, Inc. (876754375)
Trademark Results [Real Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REAL RELIEF 87936596 not registered Dead/Abandoned |
7631103 CANADA INC. 2018-05-25 |
 REAL RELIEF 86058868 not registered Dead/Abandoned |
HOMEOLAB USA INC. 2013-09-09 |
 REAL RELIEF 75317272 2301275 Dead/Cancelled |
Pfizer Inc. 1997-06-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.