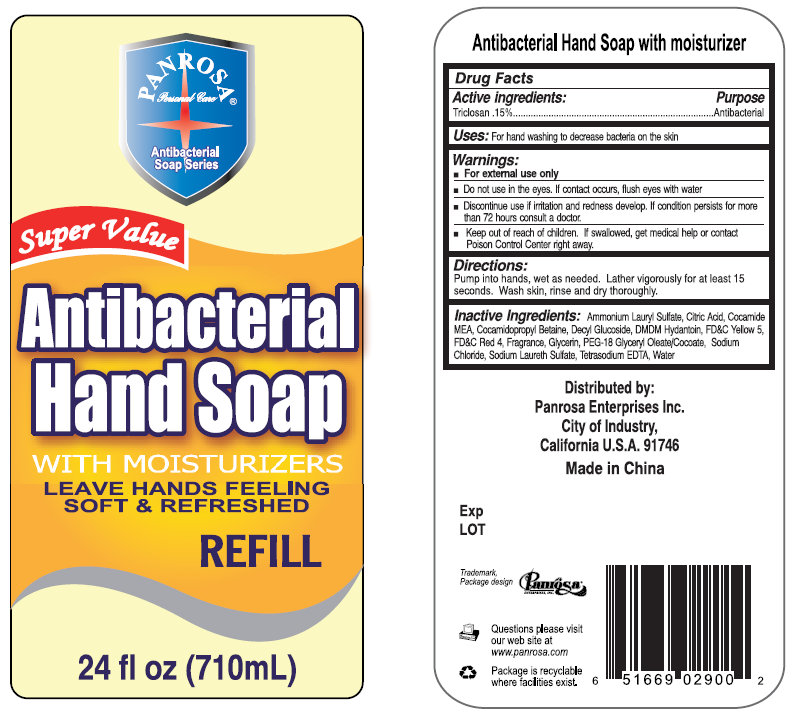
Panrosa Antibacterial Hand Soap With Moisturizers
Panrosa Antibacterial Hand With Moisturizers by
Drug Labeling and Warnings
Panrosa Antibacterial Hand With Moisturizers by is a Otc medication manufactured, distributed, or labeled by Panrosa Enterprises, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PANROSA ANTIBACTERIAL HAND WITH MOISTURIZERS- triclosan soap
Panrosa Enterprises, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Panrosa Antibacterial Hand Soap With Moisturizers
Warnings
Warnings:
For external use only
Do not use in the eyes. If contact occurs, flush eyes with water
Discontinue use if irritation and redness develop. If condition persists for more than 72 hours consult a doctor.
Directions
Pump into hands, wet as needed. Lather vigorously for at least 15 seconds. Wash skin, rinse and dry thoroughly.
Inactive Ingredient
Ammonium Lauryl Sulfate, Citric Acid, Cocamide MEA, Cocamidopropyl Betaine, Decyl Glucoside, DMDM Hydantoin, FD C Yellow 5, FD C Red 4, Fragrance, Glycerin, PEG-18 Glyceryl Oleate/Cocoate, Sodium Chloride, Sodium Laureth Sulfate, Tetrasodium EDTA, Water
Distributed by:
Panrosa Enterprises Inc.
City of Industry,
California U.S.A. 91746
Made in China
Questions please visit our web site at www.panrosa.com
Package is recyclable where facilities exist.
| PANROSA ANTIBACTERIAL HAND WITH MOISTURIZERS
triclosan soap |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Panrosa Enterprises, Inc. (859957578) |