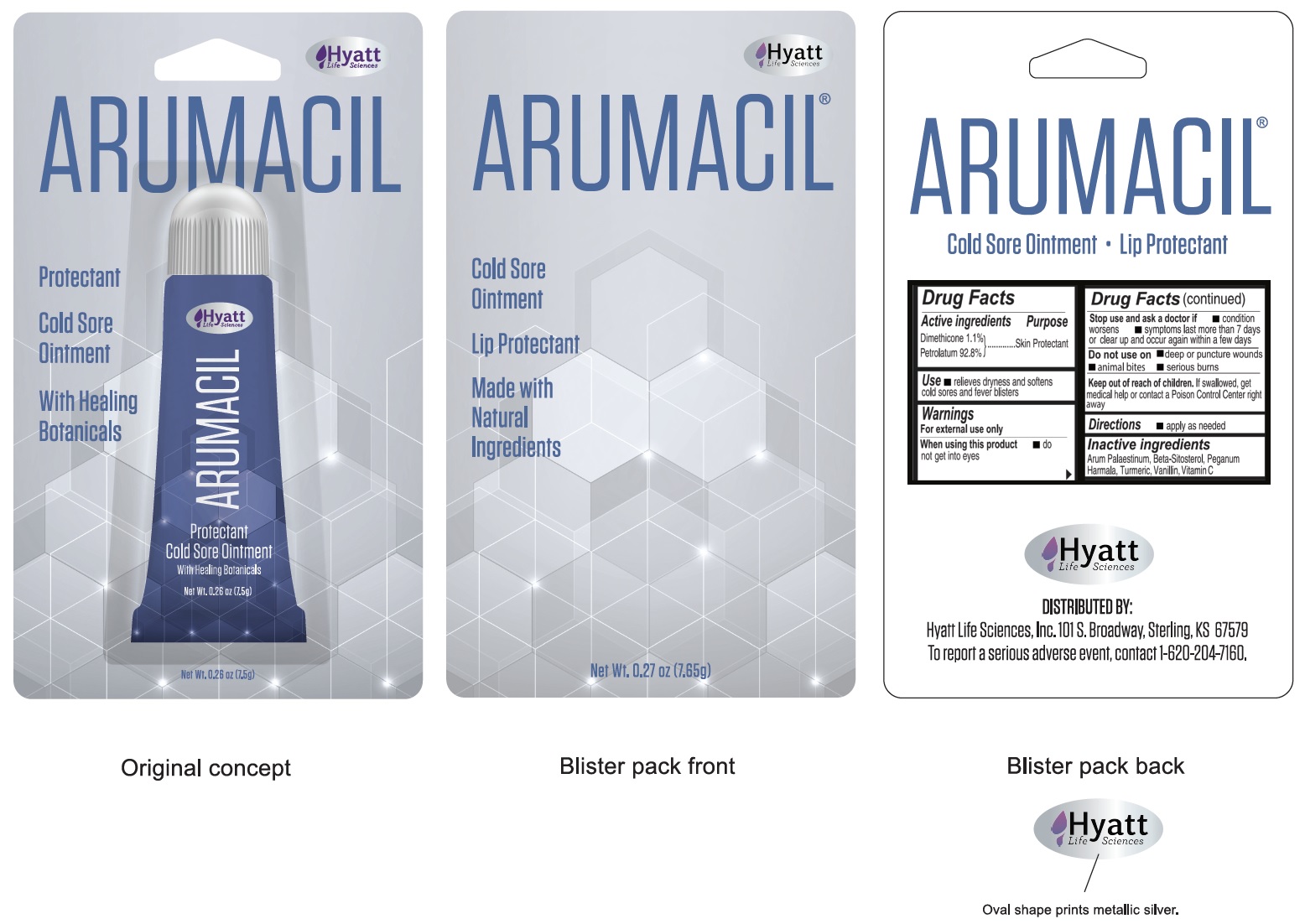
Arumacil Protectant Cold Sore Ointment
Arumacil Protectant Cold Sore by
Drug Labeling and Warnings
Arumacil Protectant Cold Sore by is a Otc medication manufactured, distributed, or labeled by Hyatt Life Sciences, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ARUMACIL PROTECTANT COLD SORE- dimethicone, petrolatum ointment
Hyatt Life Sciences, Inc.
----------
Arumacil Protectant Cold Sore Ointment
Warnings
For external use only
| ARUMACIL PROTECTANT COLD SORE
dimethicone, petrolatum ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hyatt Life Sciences, Inc. (082958972) |
Revised: 11/2023
Document Id: 09ccb37b-78de-b510-e063-6294a90a48d5
Set id: b7b38985-a96b-22c5-e053-2995a90a3f9c
Version: 3
Effective Time: 20231110