LEVOCETIRIZINE DIHYDROCHLORIDE tablet
Levocetirizine Dihydrochloride by
Drug Labeling and Warnings
Levocetirizine Dihydrochloride by is a Prescription medication manufactured, distributed, or labeled by Bryant Ranch Prepack. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use levocetirizine dihydrochloride safely and effectively. See full prescribing information for levocetirizine dihydrochloride.
Levocetirizine Dihydrochloride Tablets
Initial U.S. Approval: 1995
RECENT MAJOR CHANGES
Warningsand Precautions, Urinary Retention (5.2) [09/2012]
INDICATIONS AND USAGE
Levocetirizine dihydrochloride is a histamine H11-receptor antagonist indicated for:
The treatment of the uncomplicated skin manifestations of chronic idiopathic urticaria (1.31.3)DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Immediate release breakable (scored) tablets, 5 mg (33)CONTRAINDICATIONS
Patients with a known hypersensitivity to levocetirizine or any of the ingredients of levocetirizine
dihydrochloride tablets or to cetirizine (44)
Patients with end-stage renal disease at less than 10 mL/min creatinine clearance or patients undergoing
hemodialysis (44)
Children 6 to 11 years of age with renal impairment (44)
WARNINGS AND PRECAUTIONS
Avoid engaging in hazardous occupations requiring complete mental alertness such as driving or
operating machinery when taking levocetirizine dihydrochloride (5.1).
Avoid concurrent use of alcohol or other central nervous system depressants with levocetirizine dihydrochloride (5.1).
Use with caution in patients with predisposing factors of urinary retention (e.g. spinal cord lesion, prostatic hyperplasia). Discontinue levocetirizine dihydrochloride if urinary retention occurs (5.2). (5)ADVERSE REACTIONS
The most common adverse reactions (rate ≥2% and > placebo) were somnolence, nasopharyngitis, fatigue, dry mouth, and pharyngitis in subjects 12 years of age and older, and pyrexia, somnolence, cough, and epistaxis in children 6 to 12 years of age. (6.16.1).
To report SUSPECTED ADVERSE REACTIONS, contact Hetero Labs Limited at 866-495-1995 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.To report SUSPECTED ADVERSE REACTIONS, contact Hetero Labs Limited at 866-495-1995 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.USE IN SPECIFIC POPULATIONS
Renal Impairment
Because levocetirizine dihydrochloride is substantially excreted by the kidneys, the risk of adverse
reactions to this drug may be greater in patients with impaired renal function (8.68.6 and 12.312.3).
Pediatric Use
Do not exceed the recommended doses of 2.5 mg once daily in children 6 to 11 years. Systemic
exposure with this dose in pediatric age groups is comparable to that from a 5 mg once daily dose
in adults. (12.312.3).
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.’s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.’s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS & USAGE
1.3 Chronic Idiopathic Urticaria
2 DOSAGE & ADMINISTRATION
2.1 Adults and Children 12 Years of Age and Older
2.2 Children 6 to 11 Years of Age
2.3 Children 6 months to 5 Years of Age
2.4 Dose Adjustment for Renal and Hepatic Impairment
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
4.1 Patients with known hypersensitivity
4.2 Patients with end-stage renal disease
4.3 Pediatric patients with impaired renal function
5 WARNINGS AND PRECAUTIONS
5.1 Somnolence
5.2 Urinary Retension
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Antipyrine, Azithromycin, Cimetidine, Erythromycin, Ketoconazole, Theophylline, and Pseudoephedrine
7.2 Ritonavir
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
13.2 Animal Pharmacology & OR Toxicology
14 CLINICAL STUDIES
14.2 Chronic Idiopathic Urticaria
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Somnolence
17.2 Concomitant Use of Alcohol and other Central Nervous System Depressants
17.3 Dosing of Levocetirizine Dihydrochloride
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS & USAGE
1.3 Chronic Idiopathic Urticaria
Levocetirizine dihydrochloride tablets are indicated for the treatment of the uncomplicated skin manifestations of chronic idiopathic urticaria in adults and children 6 years of age and older.
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
-
2 DOSAGE & ADMINISTRATION
Levocetirizine dihydrochloride tablets are available as 5 mg breakable (scored) tablets, allowing for the administration of 2.5 mg, if needed. Levocetirizine dihydrochloride tablets can be taken without regard to food consumption.2.1 Adults and Children 12 Years of Age and Older
The recommended dose of levocetirizine dihydrochloride tablets are 5 mg (1 tablet) once daily in the evening. Some patients may be adequately controlled by 2.5 mg (1/2 tablet) once daily in the evening.2.2 Children 6 to 11 Years of Age
The recommended dose of levocetirizine dihydrochloride tablets are 2.5 mg (1/2 tablet) once daily in the evening. The 2.5 mg dose should not be exceeded because the systemic exposure with 5 mg is approximately twice that of adults [see Clinical Pharmacology (12.3 )Clinical Pharmacology (12.3 )Clinical Pharmacology (12.3 )].2.3 Children 6 months to 5 Years of Age
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.2.4 Dose Adjustment for Renal and Hepatic Impairment
In adults and children 12 years of age and older with:
Mild renal impairment (creatinine clearance [CLCRCR] = 50 to 80 mL/min): a dose of 2.5 mg once daily is recommended;
Moderate renal impairment (CLCRCR = 30 to 50 mL/min): a dose of 2.5 mg once every other day is recommended;
Severe renal impairment (CLCRCR = 10 to 30 mL/min): a dose of 2.5 mg twice weekly (administered once every 3 to 4 days) is recommended;
End-stage renal disease patients (CLCRCR < 10 mL/min) and patients undergoing hemodialysis should not receive levocetirizine dihydrochloride
tablets.
No dose adjustment is needed in patients with solely hepatic impairment. In patients with both hepatic impairment and renal impairment, adjustment of the dose is recommended.
- 3 DOSAGE FORMS & STRENGTHS
-
4 CONTRAINDICATIONS
The use of levocetirizine dihydrochloride is contraindicated in:4.1 Patients with known hypersensitivity
Patients with known hypersensitivity to levocetirizine or any of the ingredients of levocetirizine dihydrochloride tablets, or to cetirizine. Observed reactions range from urticaria to anaphylaxis [see Adverse Reactions (6.2 )].
4.2 Patients with end-stage renal disease
Patients with end-stage renal disease (CLCRCR < 10 mL/min) and patients undergoing hemodialysis.4.3 Pediatric patients with impaired renal function
Children 6 to 11 years of age with impaired renal function.
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients. -
5 WARNINGS AND PRECAUTIONS
5.1 Somnolence
In clinical trials the occurrence of somnolence, fatigue, and asthenia has been reported in some patients under therapy with levocetirizine dihydrochloride. Patients should be cautioned against engaging in hazardous occupations requiring complete mental alertness, and motor coordination such as operating machinery or driving a motor vehicle after ingestion of levocetirizine dihydrochloride. Concurrent use of levocetirizine dihydrochloride with alcohol or other central nervous system depressants should be avoided because additional reductions in alertness and additional impairment of central nervous system performance may occur.5.2 Urinary Retension
Urinary retention has been reported post-marketing with levocetirizine dihydrochloride. Levocetirizine dihydrochloride should be used with caution in patients with predisposing factors of urinary retention (e.g. spinal cord lesion, prostatic hyperplasia) as levocetirizine dihydrochloride may increase the risk of urinary retention. Discontinue levocetirizine dihydrochloride if urinary retention occurs.
Urinary retention has been reported post-marketing with levocetirizine dihydrochloridelevocetirizine dihydrochloride. Levocetirizine dihydrochloride should be used with caution in patients with predisposing factors of urinary retention (e.g. spinal cord lesion, prostatic hyperplasia) as levocetirizine dihydrochloride may increase the risk of urinary retention. Discontinue levocetirizine dihydrochloride if urinary retention occurs.
-
6 ADVERSE REACTIONS
Use of levocetirizine dihydrochloride has been associated with somnolence, fatigue, and astheniaand urinary retention [see Warnings and Precautions (5 )Warnings and Precautions (5 )Warnings and Precautions (5 )].6.1 Clinical Trials Experience
The safety data described below reflect exposure to levocetirizine dihydrochloride in 12 controlled clinical trials of 1 week to 6 months duration.
The short-term (exposure up to 6 weeks) safety data for adults and adolescents are based upon eight clinical trials in which 1896 patients (825 males and 1071 females aged 12 years and older) were treated with levocetirizine dihydrochloride 2.5, 5, or 10 mg once daily in the evening.
The short-term safety data from pediatric patients are based upon two clinical trials in which 243 children (162 males and 81 females 6 to 12 years of age) were treated with levocetirizine dihydrochloride 5 mg once daily for 4 to 6 weeks.
The long-term (exposure of 4 or 6 months) safety data in adults and adolescents are based upon two clinical trials in which 428 patients (190 males and 238 females) were exposed to treatment with levocetirizine dihydrochloride 5 mg once daily.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trial of another drug and may not reflect the rates observed in practice.
Adults and Adolescents 12 years of Age and Older Adults and Adolescents 12 years of Age and Older Adults and Adolescents 12 years of Age and Older
In studies up to 6 weeks in duration, the mean age of the adult and adolescent patients was 32 years, 44% of the patients were men and 56% were women, and the large majority (more than 90%) was Caucasian.
In these trials 43% and 42% of the subjects in the levocetirizine dihydrochloride 2.5 mg and 5 mg groups, respectively, had at least one adverse event compared to 43% in the placebo group.
In placebo-controlled trials of 1 to 6 weeks in duration, the most common adverse reactions were somnolence, nasopharyngitis, fatigue, dry mouth, and pharyngitis, and most were mild to moderate in intensity. Somnolence with levocetirizine dihydrochloride showed dose ordering between tested doses of 2.5, 5 and 10 mg and was the most common adverse reaction leading to discontinuation (0.5%).
Table 1 lists adverse reactions that were reported in greater than or equal to 2% of subjects aged 12 years and older exposed to levocetirizine dihydrochloride 2.5 mg or 5 mg in eight placebo-controlled clinical trials and that were more common with levocetirizine dihydrochloride than placebo.
Table 1Table 1 Adverse Reactions Reported in ≥ 2%* of Subjects Aged 12 Years and Older Exposed to Levocetirizine Dihydrochloride 2.5 mg Adverse Reactions Reported in ≥ 2%* of Subjects Aged 12 Years and Older Exposed to Levocetirizine Dihydrochloride 2.5 mg or 5 mg Once Daily in Placebo-Controlled Clinical Trials 1 to 6 Weeks in Durationor 5 mg Once Daily in Placebo-Controlled Clinical Trials 1 to 6 Weeks in Duration
Adverse
ReactionsLevocetirizine dihydrochloride
2.5 mg
(n = 421)Levocetirizine dihydrochloride
5 mg
(n = 1070)Placebo
(n = 912)Somnolence 22 (5%) 61 (6%) 16 (2%) Nasopharyngitis 25 (6%) 40 (4%) 28 (3%) Fatigue 5 (1%) 46 (4%) 20 (2%) Dry Mouth 12 (3%) 26 (2%) 11 (1%) Pharyngitis 10 (2%) 12 (1%) 9 (1%)
* Rounded to the closest unit percentage
Additional adverse reactions of medical significance observed at a higher incidence than in placebo in adults and adolescents aged 12 years and older exposed to levocetirizine dihydrochloride are syncope (0.2%) and weight increased (0.5%).
Pediatric Patients 6 to 12 Years of Age Pediatric Patients 6 to 12 Years of Age Pediatric Patients 6 to 12 Years of Age
A total of 243 pediatric patients 6 to 12 years of age received levocetirizine dihydrochloride 5 mg once daily in two short-term placebo controlled double-blind trials. The mean age of the patients was 9.8 years, 79 (32%) were 6 to 8 years of age, and 50% were Caucasian. Table 2 lists adverse reactions that were reported in greater than or equal to 2% of subjects aged 6 to 12 years exposed to levocetirizine dihydrochloride 5 mg in placebo-controlled clinical trials and that were more common with levocetirizine dihydrochloride than placebo.
Table 2 Adverse Reactions Reported in ≥2%* of Subjects Aged 6 to 12 Years Exposed to Levocetirizine Dihydrochloride 5 mg Once Daily in Placebo-Controlled Clinical Trials 4 and 6 Weeks in Duration
Table 2 Adverse Reactions Reported in ≥2%* of Subjects Aged 6 to 12 Years Exposed to Levocetirizine Dihydrochloride 5 mg Once Daily in Placebo-Controlled Clinical Trials 4 and 6 Weeks in Duration
Adverse
ReactionsLevocetirizine Dihydrochloride5 mg
(n = 243)Placebo (n = 240) Pyrexia 10 (4%) 5 (2%) Cough 8 (3%) 2 (<1%) Somnolence 7 (3%) 1 (<1%) Epistaxis 6 (2%) 1 (<1%)
* Rounded to the closest unit percentageClinical trial information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
Clinical trial information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.Clinical trial information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
Long-Term Clinical Trials Experience
Long-Term Clinical Trials Experience
In two controlled clinical trials, 428 patients (190 males and 238 females) aged 12 years and older were treated with levocetirizine dihydrochloride 5 mg once daily for 4 or 6 months. The patient characteristics and the safety profile were similar to that seen in the short-term studies. Ten (2.3%) patients treated with levocetirizine dihydrochloride discontinued because of somnolence, fatigue or asthenia compared to 2 (<1%) in the placebo group.
There are no long term clinical trials in children below 12 years of age with chronic idiopathic urticaria.
Laboratory Test Abnormalities Laboratory Test Abnormalities Laboratory Test Abnormalities
Elevations of blood bilirubin and transaminases were reported in <1% of patients in the clinical trials. The elevations were transient and did not lead to discontinuation in any patient.6.2 Post-Marketing Experience
In addition to the adverse reactions reported during clinical trials and listed above, adverse events have also been identified during post-approval use of levocetirizine dihydrochloride. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Adverse events of hypersensitivity and anaphylaxis, increased appetite, angioedema, fixed drug eruption, pruritus, rash and urticaria, convulsion, paraesthesia, dizziness, tremor, dysgeusia, vertigo, aggression and agitation, hallucinations, depression, insomnia, suicidal ideation, visual disturbances, blurred vision, palpitations, tachycardia, dyspnea, nausea, vomiting, hepatitis, dysuria, urinary retention, myalgia, and edema have been reported.
Besides these events reported under treatment with levocetirizine dihydrochloride, other potentially severe adverse events have been reported from the post-marketing experience with cetirizine. Since levocetirizine is the principal pharmacologically active component of cetirizine, one should take into account the fact that the following adverse events could also potentially occur under treatment with levocetirizine dihydrochloride: orofacial dyskinesia, severe hypotension, cholestasis, glomerulonephritis, and still birth.
-
7 DRUG INTERACTIONS
In vitroIn vitro data indicate that levocetirizine is unlikely to produce pharmacokinetic interactions through inhibition or induction of liver drug-metabolizing enzymes. No in vivoin vivo drug-drug interaction studies have been performed with levocetirizine. Drug interaction studies have been performed with racemic cetirizine.7.1 Antipyrine, Azithromycin, Cimetidine, Erythromycin, Ketoconazole, Theophylline, and Pseudoephedrine
Pharmacokinetic interaction studies performed with racemic cetirizine demonstrated that cetirizine did not interact with antipyrine, pseudoephedrine, erythromycin, azithromycin, ketoconazole, and cimetidine. There was a small decrease (~16%) in the clearance of cetirizine caused by a 400 mg dose of theophylline. It is possible that higher theophylline doses could have a greater effect. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Pregnancy Category B
There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, levocetirizine dihydrochloride should be used during pregnancy only if clearly needed.
Teratogenic Effects:
Teratogenic Effects:
In rats and rabbits, levocetirizine was not teratogenic at oral doses approximately 320 and 390, respectively, times the maximum recommended daily oral dose in adults on a mg/m22 basis.8.3 Nursing Mothers
No peri- and post-natal animal studies have been conducted with levocetirizine. In mice, cetirizine caused retarded pup weight gain during lactation at an oral dose in dams that was approximately 40 times the maximum recommended daily oral dose in adults on a mg/m2 2 basis. Studies in beagle dogs indicated that approximately 3% of the dose of cetirizine was excreted in milk. Cetirizine has been reported to be excreted in human breast milk. Because levocetirizine is also expected to be excreted in human milk, use of levocetirizine dihydrochloride in nursing mothers is not recommended.8.4 Pediatric Use
The recommended dose of levocetirizine dihydrochloride for the treatment of the uncomplicated skin manifestations of chronic idiopathic urticaria in patients 12 to 17 years of age is based on extrapolation of efficacy from adults 18 years of age and older [see Clinical Studies (14 )Clinical Studies (14 )Clinical Studies (14 )].
The recommended dose of levocetirizine dihydrochloride in patients 6 to 11 years of age for the treatment of the symptoms of chronic idiopathic urticaria is based on cross-study of the systemic exposure of levocetirizine dihydrochloride in adults and pediatric patients and on the safety profile of levocetirizine dihydrochloride in both adult and pediatric patients at doses equal to or higher than the recommended dose for patients 6 to 11 years of age.
The safety of levocetirizine dihydrochloride 5 mg once daily was evaluated in 243 pediatric patients 6 to 12 years of age in two placebo-controlled clinical trials lasting 4 and 6 weeks. [see Adverse Reactions (6.1 )Adverse Reactions (6.1 )Adverse Reactions (6.1 )].
The effectiveness of levocetirizine dihydrochloride 2.5 mg once daily (6 to 11 years of age) for the treatment of the chronic idiopathic urticaria is supported by the extrapolation of demonstrated efficacy of levocetirizine dihydrochloride 5 mg once daily in patients 12 years of age and older based on the pharmacokinetic comparison between adults and children.
Cross-study comparisons indicate that administration of a 5 mg dose of levocetirizine dihydrochloride to 6 to 12 year old pediatric patients resulted in about 2-fold the systemic exposure (AUC) observed when 5 mg of levocetirizine dihydrochloride was administered to healthy adults. Therefore, in children 6 to 11 years of age the recommended dose of 2.5 mg once daily should not be exceeded. [see Dosage and Administration ( 2.2 )Dosage and Administration ( 2.2 )Dosage and Administration ( 2.2 ); Clinical Studies (14 )Clinical Studies (14 )Clinical Studies (14 ); and Clinical Pharmacology (12.3)Clinical Pharmacology (12.3)Clinical Pharmacology (12.3)].
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.8.5 Geriatric Use
Clinical studies of levocetirizine dihydrochloride for each approved indication did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.8.6 Renal Impairment
Levocetirizine dihydrochloride is known to be substantially excreted by the kidneys and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function [see Dosage and Administration (2)Dosage and Administration (2)Dosage and Administration (2) and Clinical Pharmacology (12.3 )Clinical Pharmacology (12.3 )Clinical Pharmacology (12.3 )].8.7 Hepatic Impairment
As levocetirizine is mainly excreted unchanged by the kidneys, it is unlikely that the clearance of levocetirizine is significantly decreased in patients with solely hepatic impairment [see Clinical Pharmacology (12.3 )Clinical Pharmacology (12.3 )Clinical Pharmacology (12.3 )]. -
10 OVERDOSAGE
Overdosage has been reported with levocetirizine dihydrochloride.
Symptoms of overdose may include drowsiness in adults and initially agitation and restlessness, followed by drowsiness in children. There is no known specific antidote to levocetirizine dihydrochloride. Should overdose occur, symptomatic or supportive treatment is recommended. Levocetirizine dihydrochloride is not effectively removed by dialysis, and dialysis will be ineffective unless a dialyzable agent has been concomitantly ingested.
The acute maximal non-lethal oral dose of levocetirizine was 240 mg/kg in mice (approximately 190 times the maximum recommended daily oral dose in adults, approximately 230 times the maximum recommended daily oral dose in children 6 to 11 years of age on a mg/m22 basis). In rats the maximal non-lethal oral dose was 240 mg/kg (approximately 390 times the maximum recommended daily oral dose in adults, approximately 460 times the maximum recommended daily oral dose in children 6 to 11 years of age on a mg/m22 basis).
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients. -
11 DESCRIPTION
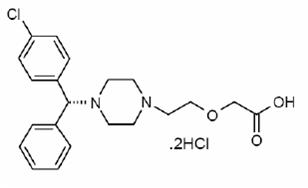
Levocetirizine dihydrochloride, the active component of levocetirizine dihydrochloride tablets, is an orally active H11-receptor antagonist. The chemical name is (R)-[2-[4-[(4-chlorophenyl) phenylmethyl]-1-piperazinyl] ethoxy] acetic acid dihydrochloride. Levocetirizine dihydrochloride is the R enantiomer of cetirizine hydrochloride, a racemic compound with antihistaminic properties. The empirical formula of levocetirizine dihydrochloride is C2121H2525ClN22O332HCl. The molecular weight is 461.82 and the chemical structure is shown below:
Levocetirizine dihydrochloride is a white, crystalline powder and is water soluble.
Levocetirizine dihydrochloride 5 mg tablets are white film coated, scored, round shaped tablets for oral administration. Inactive ingredients are: colloidal silicon dioxide, lactose monohydrate, magnesium stearate and microcrystalline cellulose and opadry white YS-1-7003 which contains hypromellose, polyethylene glycol, polysorbate 80 and titanium dioxide. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Levocetirizine, the active enantiomer of cetirizine, is an anti-histamine; its principal effects are mediated via selective inhibition of H11 receptors. The antihistaminic activity of levocetirizine has been documented in a variety of animal and human models. In vitroIn vitro binding studies revealed that levocetirizine has an affinity for the human H11-receptor 2-fold higher than that of cetirizine (Ki = 3 nmol/L vs. 6 nmol/L, respectively). The clinical relevance of this finding is unknown.12.2 Pharmacodynamics
Studies in adult healthy subjects showed that levocetirizine at doses of 2.5 mg and 5 mg inhibited the skin wheal and flare caused by the intradermal injection of histamine. In contrast, dextrocetirizine exhibited no clear change in the inhibition of the wheal and flare reaction. Levocetirizine at a dose of 5 mg inhibited the wheal and flare caused by intradermal injection of histamine in 14 pediatric subjects (aged 6 to 11 years) and the activity persisted for at least 24 hours. The clinical relevance of histamine wheal skin testing is unknown.
A QT/QTc study using a single dose of 30 mg of levocetirizine did not demonstrate an effect on the QTc interval. While a single dose of levocetirizine had no effect, the effects of levocetirizine may not be at steady state following single dose. The effect of levocetirizine on the QTc interval following multiple dose administration is unknown. Levocetirizine is not expected to have QT/QTc effects because of the results of QTc studies with cetirizine and the long post-marketing history of cetirizine without reports of QT prolongation.12.3 Pharmacokinetics
Levocetirizine exhibited linear pharmacokinetics over the therapeutic dose range in adult healthy subjects.
Absorption
Absorption
Levocetirizine is rapidly and extensively absorbed following oral administration. In adults, peak plasma concentrations are achieved 0.9 hour after administration of the oral tablet. The accumulation ratio following daily oral administration is 1.12 with steady state achieved after 2 days. Peak concentrations are typically 270 ng/mL and 308 ng/mL following a single and a repeated 5 mg once daily dose, respectively. Food had no effect on the extent of exposure (AUC) of the levocetirizine tablet, but Tmaxmax was delayed by about 1.25 hours and Cmaxmax was decreased by about 36% after administration with a high fat meal; therefore, levocetirizine can be administered with or without food.
Distribution
Distribution
The mean plasma protein binding of levocetirizine in vitro ranged from 91 to 92%, independent of concentration in the range of 90 to 5000 ng/mL, which includes the therapeutic plasma levels observed. Following oral dosing, the average apparent volume of distribution is approximately 0.4 L/kg, representative of distribution in total body water.
Metabolism
Metabolism
The extent of metabolism of levocetirizine in humans is less than 14% of the dose and therefore differences resulting from genetic polymorphism or concomitant intake of hepatic drug metabolizing enzyme inhibitors are expected to be negligible. Metabolic pathways include aromatic oxidation, N- and O-dealkylation, and taurine conjugation. Dealkylation pathways are primarily mediated by CYP 3A4 while aromatic oxidation involves multiple and/or unidentified CYP isoforms.
Elimination
Elimination
The plasma half-life in adult healthy subjects was about 8 to 9 hours after administration of oral tablets, and the mean oral total body clearance for levocetirizine was approximately 0.63 mL/kg/min. The major route of excretion of levocetirizine and its metabolites is via urine, accounting for a mean of 85.4% of the dose. Excretion via feces accounts for only 12.9% of the dose. Levocetirizine is excreted both by glomerular filtration and active tubular secretion. Renal clearance of levocetirizine correlates with that of creatinine clearance. In patients with renal impairment the clearance of levocetirizine is reduced [seeDosage and Administration (2.3 )Dosage and Administration (2.3 )Dosage and Administration (2.3 )].
Drug Interaction Studies Drug Interaction Studies
In vitroIn vitro data on metabolite interaction indicate that levocetirizine is unlikely to produce, or be subject to metabolic interactions. Levocetirizine at concentrations well above Cmaxmax level achieved within the therapeutic dose ranges is not an inhibitor of CYP isoenzymes 1A2, 2C9, 2C19, 2A1, 2D6, 2E1, and 3A4, and is not an inducer of UGT1A or CYP isoenzymes 1A2, 2C9 and 3A4.
No formal in vivoin vivo drug interaction studies have been performed with levocetirizine. Studies have been performed with the racemic cetirizine [see Drug Interactions (7)Drug Interactions (7)Drug Interactions (7)].
Pediatric Patients Pediatric Patients
Data from a pediatric pharmacokinetic study with oral administration of a single dose of 5 mg levocetirizine in 14 children age 6 to 11 years with body weight ranging between 20 and 40 kg show that Cmaxmax and AUC values are about 2-fold greater than that reported in healthy adult subjects in a cross-study comparison. The mean Cmax was 450 ng/mL, occurring at a mean time of 1.2 hours, weight-normalized, total body clearance was 30% greater, and the elimination half-life 24% shorter in this pediatric population than in adults.
Dedicated pharmacokinetic studies have not been conducted in pediatric patients younger than 6 years of age.
Pharmacokinetic information in pediatric patients (age 1 to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.Pharmacokinetic information in pediatric patients (age 1 to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.
Geriatric Patients Geriatric Patients
Limited pharmacokinetic data are available in elderly subjects. Following once daily repeat oral administration of 30 mg levocetirizine for 6 days in 9 elderly subjects (65 to 74 years of age), the total body clearance was approximately 33% lower compared to that in younger adults. The disposition of racemic cetirizine has been shown to be dependent on renal function rather than on age. This finding would also be applicable for levocetirizine, as levocetirizine and cetirizine are both predominantly excreted in urine. Therefore, the levocetirizine dihydrochloride dose should be adjusted in accordance with renal function in elderly patients [see Dosage and Administration (2)Dosage and Administration (2)Dosage and Administration (2)].
Gender
Gender
Pharmacokinetic results for 77 patients (40 men, 37 women) were evaluated for potential effect of gender. The half-life was slightly shorter in women (7.08 ± 1.72 hr) than in men (8.62 ± 1.84 hr); however, the body weight-adjusted oral clearance in women (0.67 ± 0.16 mL/min/kg) appears to be comparable to that in men (0.59 ± 0.12 mL/min/kg). The same daily doses and dosing intervals are applicable for men and women with normal renal function.
Race
Race
The effect of race on levocetirizine has not been studied. As levocetirizine is primarily renally excreted, and there are no important racial differences in creatinine clearance, pharmacokinetic characteristics of levocetirizine are not expected to be different across races. No race-related differences in the kinetics of racemic cetirizine have been observed.
Renal Impairment
Renal Impairment
Levocetirizine exposure (AUC) exhibited 1.8-, 3.2-, 4.3-, and 5.7-fold increase in mild, moderate, severe, renal impaired, and end-stage renal disease patients, respectively, compared to healthy subjects. The corresponding increases of half-life estimates were 1.4-, 2.0-, 2.9-, and 4-fold, respectively.
The total body clearance of levocetirizine after oral dosing was correlated to the creatinine clearance and was progressively reduced based on severity of renal impairment. Therefore, it is recommended to adjust the dose and dosing intervals of levocetirizine based on creatinine clearance in patients with mild, moderate, or severe renal impairment. In end-stage renal disease patients (CLCR < 10 mL/min) levocetirizine is contraindicated. The amount of levocetirizine removed during a standard 4-hour hemodialysis procedure was <10%.
The dosage of levocetirizine dihydrochloride should be reduced in patients with mild renal impairment. Both the dosage and frequency of administration should be reduced in patients with moderate or severe renal impairment [see Dosage and Administration (2.4 )Dosage and Administration (2.4 )Dosage and Administration (2.4 )Dosage and Administration (2.4 )].
Hepatic Impairment
Hepatic Impairment
Levocetirizine dihydrochloride has not been studied in patients with hepatic impairment. The non-renal clearance (indicative of hepatic contribution) was found to constitute about 28% of the total body clearance in healthy adult subjects after oral administration.
As levocetirizine is mainly excreted unchanged by the kidney, it is unlikely that the clearance of levocetirizine is significantly decreased in patients with solely hepatic impairment [see Dosage and Administration (2 )Dosage and Administration (2 )Dosage and Administration (2 )]. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
No carcinogenicity studies have been performed with levocetirizine. However, evaluation of cetirizine carcinogenicity studies are relevant for determination of the carcinogenic potential of levocetirizine. In a 2-year carcinogenicity study, in rats, cetirizine was not carcinogenic at dietary doses up to 20 mg/kg (approximately 15 times the maximum recommended daily oral dose in adults, approximately10 times the maximum recommended daily oral dose in children 6 to 11 years of age on a mg/m2 2 basis). In a 2-year carcinogenicity study in mice, cetirizine caused an increased incidence of benign hepatic tumors in males at a dietary dose of 16 mg/kg (approximately 6 times the maximum recommended daily oral dose in adults, approximately 4 times the maximum recommended daily oral dose in children 6 to 11 years of age, on a mg/m22 basis). No increased incidence of benign tumors was observed at a dietary dose of 4 mg/kg (approximately 2 times the maximum recommended daily oral dose in adults, equivalent to the maximum recommended daily oral dose in children 6 to 11 years of age on a mg/m22 basis). The clinical significance of these findings during long-term use of levocetirizine dihydrochloride is not known.
Levocetirizine was not mutagenic in the Ames test, and not clastogenic in the human lymphocyte assay, the mouse lymphoma assay, and in vivo micronucleus test in mice.
In a fertility and general reproductive performance study in mice, cetirizine did not impair fertility at an oral dose of 64 mg/kg (approximately 25 times the recommended daily oral dose in adults on a mg/m² basis).
PediatricPediatricuse information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.13.2 Animal Pharmacology & OR Toxicology
Reproductive Toxicology Studies
In rats and rabbits, levocetirizine was not teratogenic at oral doses up to 200 and 120 mg/kg, respectively, (approximately 320 and 390, respectively, times the maximum recommended daily oral dose in adults on a mg/m22 basis).
In mice, cetirizine caused retarded pup weight gain during lactation at an oral dose in dams of 96 mg/kg (approximately 40 times the maximum recommended daily oral dose in adults on a mg/m22 basis). -
14 CLINICAL STUDIES
14.2 Chronic Idiopathic Urticaria
Adult Patients 18 Years of Age and Older Adult Patients 18 Years of Age and Older Adult Patients 18 Years of Age and Older
The efficacy of levocetirizine dihydrochloride for the treatment of the uncomplicated skin manifestations of chronic idiopathic urticaria was evaluated in two multi-center, randomized, placebo-controlled, double-blind clinical trials of 4 weeks duration in adult patients 18 to 85 years of age with chronic idiopathic urticaria. The two trials included one 4-week dose-ranging trial and one 4-week single-dose level efficacy trial. These trials included 423 patients (139 males and 284 females). Most patients (>90%) were Caucasian and the mean age was 41. Of these patients, 146 received levocetirizine dihydrochloride 5 mg once daily in the evening. Efficacy was assessed based on patient recording of pruritus severity on a severity score of 0 to 3 (0 = none to 3 = severe). The primary efficacy endpoint was the mean reflective pruritus severity score over the first week and over the entire treatment period. Additional efficacy variables were the instantaneous pruritus severity score, the number and size of wheals, and duration of pruritus.
The dose-ranging trial was conducted to evaluate the efficacy of levocetirizine dihydrochloride 2.5, 5, and 10 mg once daily in the evening. In this trial, each of the three doses of levocetirizine dihydrochloride demonstrated greater decrease in the reflective pruritus severity score than placebo and the difference was statistically significant for all three doses (see Table 3).
The single dose level trial evaluated the efficacy of levocetirizine dihydrochloride 5 mg once daily in the evening compared to placebo in patients with chronic idiopathic urticaria over a 4-week treatment period. Levocetirizine dihydrochloride 5 mg demonstrated a greater decrease from baseline in the reflective pruritus severity score than placebo and the difference from placebo was statistically significant.
Duration of pruritus, number and size of wheals, and instantaneous pruritus severity score also showed significant improvement over placebo. The significant improvement in the instantaneous pruritus severity score over placebo confirmed end of dosing interval efficacy (see Table 3).
Table 3 Mean Reflective Pruritus Severity Score in Chronic Idiopathic Urticaria Trials
Treatment
N
Baseline
On Treatment Adjusted Mean
Difference from Placebo
Estimate95% CI p-value Dose-Ranging Trial - Reflective pruritis severity score
Levocetrizine dihydrochloride 2.5 mg
69
2.08
1.02
0.82(0.58, 1.06) <0.001
Levocetrizine dihydrochloride
5 mg
62
2.07
0.92
0.91(0.66, 1.16)
<0.001
Levocetrizine dihydrochloride
10 mg
55
2.04
0.73
1.11(0.85, 1.37) <0.001
Placebo
60
2.25
1.84
Chronic Idiopathic Urticaria Trial - Reflective pruritis severity score
Levocetrizine dihydrochloride
5 mg80 2.07 0.94 0.62 (0.38, 0.86) <0.001 Placebo 82 2.06 1.56 Pediatric Patients
There are no clinical efficacy trials in pediatric patients with chronic idiopathic urticaria [see Use in Specific Populations (8.4 )Use in Specific Populations (8.4 )Use in Specific Populations (8.4 )]. - 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
17.1 Somnolence
Caution patients against engaging in hazardous occupations requiring complete mental alertness, and motor coordination such as operating machinery or driving a motor vehicle after ingestion of levocetirizine dihydrochloride.17.2 Concomitant Use of Alcohol and other Central Nervous System Depressants
Instruct patients to avoid concurrent use of levocetirizine dihydrochloride with alcohol or other central nervous system depressants because additional reduction in mental alertness may occur.
17.3 Dosing of Levocetirizine Dihydrochloride
Do not exceed the recommended daily dose in adults and adolescents 12 years of age and older of 5 mg once daily in the evening. In children 6 to 11 years of age the recommended dose is 2.5 mg once daily in the evening. Advise patients to not ingest more than the recommended dose of levocetirizine dihydrochloride because of the increased risk of somnolence at higher doses.
Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.Pediatric use information in pediatric patients (age 6 months to 5 years) is approved for UCB Inc.’s levocetirizine dihydrochloride drug product labeling. However, due to UCB Inc.‘s marketing exclusivity rights; this drug product is not labeled for such use in those pediatric patients.

Manufactured for: 2019184
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854
By: HETEROHETEROTMTMHetero Labs Limited
Jeedimetla, Hyderabad – 500 055, India. - Levocetirizine Dihydrochloride 5mg Tab
-
INGREDIENTS AND APPEARANCE
LEVOCETIRIZINE DIHYDROCHLORIDE
levocetirizine dihydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71335-0789(NDC:31722-551) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOCETIRIZINE DIHYDROCHLORIDE (UNII: SOD6A38AGA) (LEVOCETIRIZINE - UNII:6U5EA9RT2O) LEVOCETIRIZINE DIHYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score 2 pieces Shape ROUND (Standard Concave) Size 6mm Flavor Imprint Code 161;H Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71335-0789-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 05/18/2018 2 NDC: 71335-0789-2 90 in 1 BOTTLE; Type 0: Not a Combination Product 05/18/2018 3 NDC: 71335-0789-3 28 in 1 BOTTLE; Type 0: Not a Combination Product 05/18/2018 4 NDC: 71335-0789-4 60 in 1 BOTTLE; Type 0: Not a Combination Product 05/18/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091264 06/29/2012 Labeler - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(71335-0789) , RELABEL(71335-0789)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
