UNITED AIRLINES UPP KIT WITH SANITIZER WIPE- alcohol, sodium monofluorophosphate
Buzz Products (HK) Co. Limited
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Ethyl Alcohol 75% (v/v)
Uses
- To help reduce bacteria on the skin.
Warnings
- For external use only.
- Do not dispose of wipe in flush toilets.
Flammable
- Keep away from fire or flame.
When using this product
- avoid contact with eyes,
- If contact occurs, rinse thoroughly with water.
Stop using and ask a doctor if
irritation or redness develops and lasts.
Keep out of reach of children
In case of accidental ingestion, get medical help or contact a poison control center immediately.
Directions
- Apply to hands or surfaces.
- No rinsing required.
- Discard after use.
Other Information
- Store below 110°F (43°C).
- May discolor certain fabrics or surfaces.
Inactive ingredients
Aqua (Water), Glycerin, Polyquaternium-37, Melaleuca Alternifolia Leaf Oil.
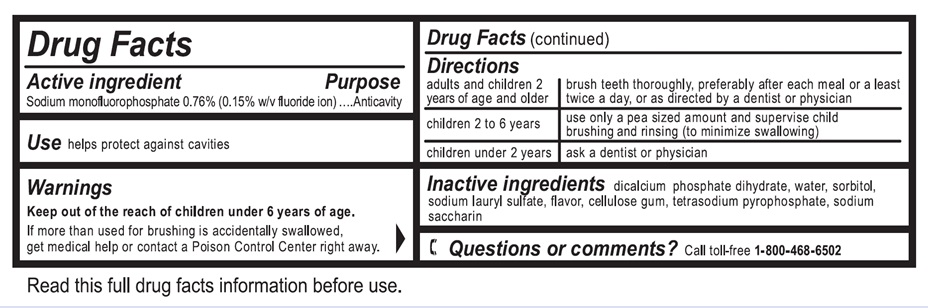
Active ingredient
Sodium monofluorophosphate 0.76% (0.15% w/v fluoride ion)
Use
helps protect against cavities
Warnings
Keep out of the reach of children under 6 years of age.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
| adults and children 2 years of age and older | brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician |
| children 2 to 6 years | use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) |
| children under 2 years | ask a dentist or physician |
Inactive ingredients
dicalcium phosphate dihydrate, water, sorbitol, sodium lauryl sulfate, flavor, cellulose gum, tetrasodium pyrophosphate, sodium saccharin
Questions or comments?
Call to-free 1-800-468-6502
Package Labeling:70402-257-01

Package Labeling:70402-256-01