PCA Skincare Active Protection Body Broad Spectrum SPF 30
Active SPF 30 by
Drug Labeling and Warnings
Active SPF 30 by is a Otc medication manufactured, distributed, or labeled by CP Skin Health Group, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ACTIVE SPF 30- broad spectrum lotion
CP Skin Health Group, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
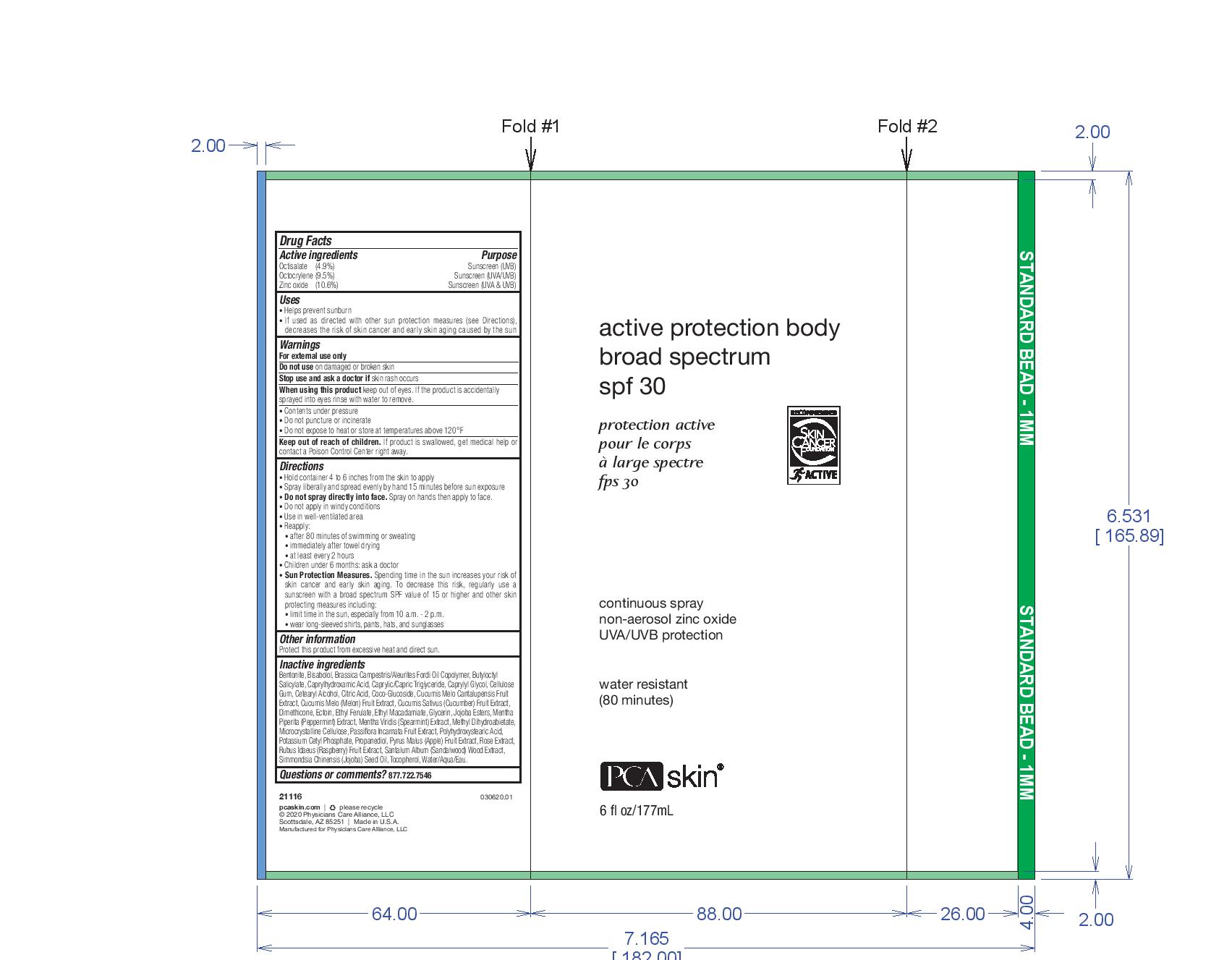
PCA Skincare Active Protection Body Broad Spectrum SPF 30
Uses
Helps prevent sunburn
If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
For external use only
Do not use on damaged or broken skin
Stop use and ask a doctor if skin rash occurs
When using this product keep out of eyes. If the product is accidentally sprayed into eyes rinse with water to remove.
Contents under pressure
Do not puncture or incinerate
Do not expose to heat or store at temperatures above 120 F
Directions
Hold container 4 to 6 inches from the skin to apply
Spray liberally and spread evenly by hand 15 minutes before sun exposure
Do not spray directly into face. Spray on hands then apply to face.
Do not apply in windy conditions
Use in a well-ventilated area
Reapply
-after 80 minutes of swimming or sweating
-immediately after towel drying
-at least every 2 hours
Children under 6 months: ask a doctor
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a borad spectrum SPF value of 15 or higher and other skin protecting measures including:
-limit time in the sun, epseically from 10 a.m. to 2 p.m.
-wear long-sleeved shirts, pants, hats, and sunglasses
Inactive Ingredients
Bentonite
Bisabolol
Brassica Campestris/Aleurites Fordi Oil Copolymer
Butyloctyl Salicylate
Caprylhydroxamic Acid
Caprylic/Capric Triglyceride
Caprylyl Glycol
Cellulose Gum
Cetearyl Alcohol
Citric Acid
Coco-Glucoside
Cucumis Melo (Melon) Fruit Extract
Cucumis Melo Cantalupensis Fruit Extract
Cucumis Sativus (Cucumber) Fruit Extract
Dimethicone
Ectoin
Ethyl Ferulate
Ethyl Macadamiate
Glycerin
Jojoba Esters
Mentha Piperita (Peppermint) Extract
Mentha Viridis (Spearmint) Extract
Methyl Dihydroabietate
Microcrystalline Cellulose
Passiflora Incarnata Fruit Extract
Polyhydroxystearic Acid
Potassium Cetyl Phosphate
Propanediol
Pyrus Malus (Apple) Fruit Extract
Rose Extract
Rubus Idaeus (Raspberry) Fruit Extract
Santalum Album (Sandalwood) Wood Extract
Simmondsia Chinensis (Jojoba) Seed Oil
Tocopherol
Water
| ACTIVE SPF 30
broad spectrum lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - CP Skin Health Group, Inc. (611921669) |