POZCOS PO CC Cream by Chang Ha Co.,Ltd / GreenCos co.,Ltd
POZCOS PO CC Cream by
Drug Labeling and Warnings
POZCOS PO CC Cream by is a Otc medication manufactured, distributed, or labeled by Chang Ha Co.,Ltd, GreenCos co.,Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
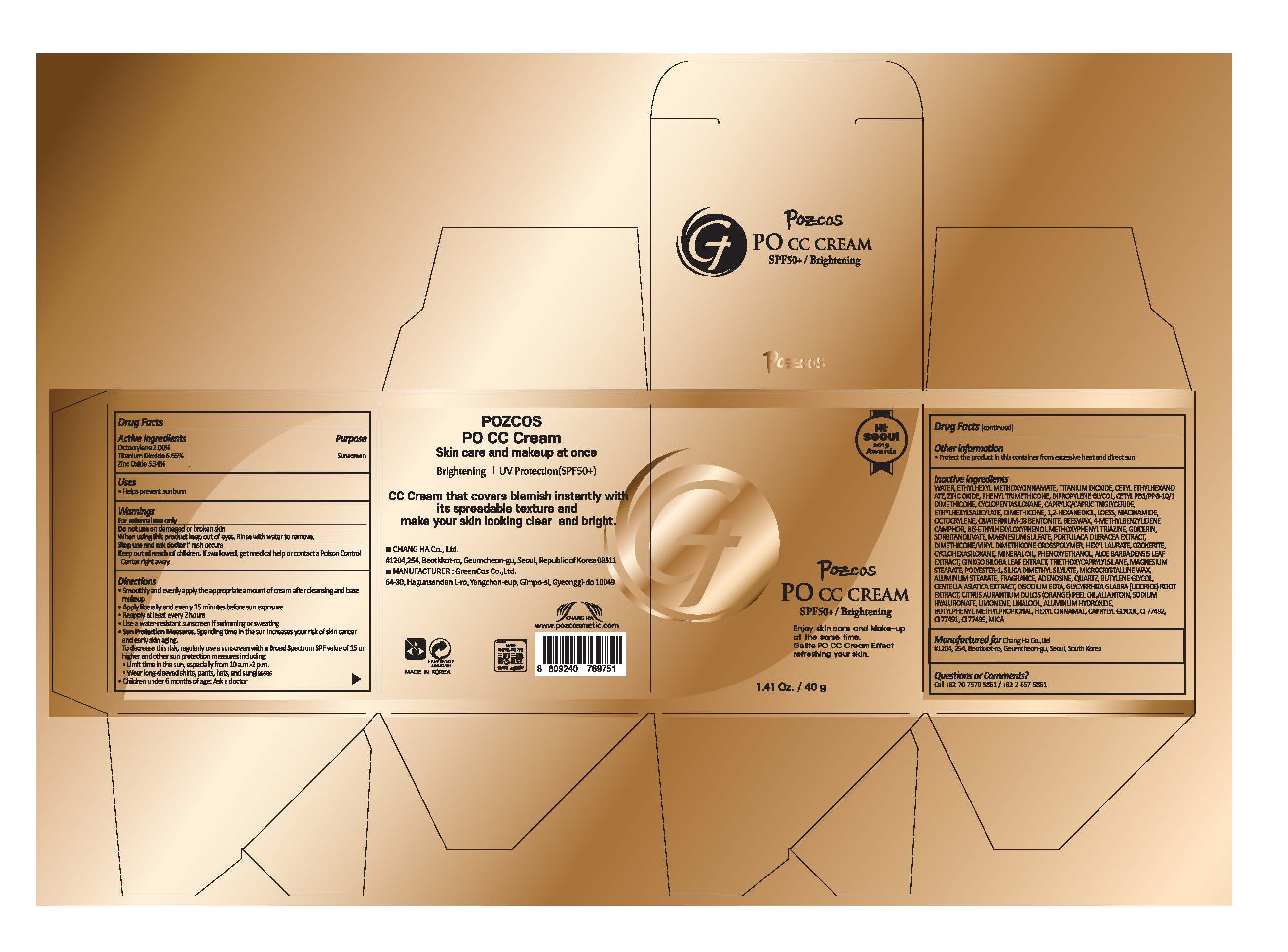
POZCOS PO CC CREAM- titanium dioxide, zinc oxide, octocrylene cream
Chang Ha Co.,Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask doctor if rash occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Smoothly and evenly apply the appropriate amount of cream after cleansing and base makeup
Apply liberally and evenly 15 minutes before sun exposure
Reapply at least every 2 hours
Use a water-resistant sunscreen if swimming or sweating
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
Limit time in the sun, especially from 10 a.m.-2 p.m.
Wear long-sleeved shirts, pants, hats, and sunglasses
Children under 6 months of age: Ask a doctor
Inactive ingredients
WATER, ETHYLHEXYL METHOXYCINNAMATE, TITANIUM DIOXIDE, CETYL ETHYLHEXANOATE, ZINC OXIDE, PHENYL TRIMETHICONE, DIPROPYLENE GLYCOL, CETYL PEG/PPG-10/1 DIMETHICONE, CYCLOPENTASILOXANE, CAPRYLIC/CAPRIC TRIGLYCERIDE, ETHYLHEXYL SALICYLATE, DIMETHICONE, 1,2-HEXANEDIOL, LOESS, NIACINAMIDE, OCTOCRYLENE, QUATERNIUM-18 BENTONITE, BEESWAX, 4-METHYLBENZYLIDENE CAMPHOR, BIS-ETHYLHEXYLOXYPHENOL METHOXYPHENYL TRIAZINE, GLYCERIN, SORBITAN OLIVATE, MAGNESIUM SULFATE, PORTULACA OLERACEA EXTRACT, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, HEXYL LAURATE, OZOKERITE, CYCLOHEXASILOXANE, MINERAL OIL, PHENOXYETHANOL, ALOE BARBADENSIS LEAF EXTRACT, GINKGO BILOBA LEAF EXTRACT, TRIETHOXYCAPRYLYLSILANE, MAGNESIUM STEARATE, POLYESTER-1, SILICA DIMETHYL SILYLATE, MICROCRYSTALLINE WAX, ALUMINUM STEARATE, FRAGRANCE, ADENOSINE, QUARTZ, BUTYLENE GLYCOL, CENTELLA ASIATICA EXTRACT, DISODIUM EDTA, GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT, CITRUS AURANTIUM DULCIS (ORANGE) PEEL OIL, ALLANTOIN, SODIUM HYALURONATE, LIMONENE, LINALOOL, ALUMINUM HYDROXIDE, BUTYLPHENYL METHYLPROPIONAL, HEXYL CINNAMAL, CAPRYLYL GLYCOL, CI 77492, CI 77491, CI 77499, MICA
| POZCOS PO CC CREAM
titanium dioxide, zinc oxide, octocrylene cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Chang Ha Co.,Ltd (690395914) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Chang Ha Co.,Ltd | 690395914 | pack(81397-201) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.