Wet Ones Antibacterial Hand Wipes Spring Bliss by Edgewell Personal Care Brands LLC
Wet Ones Antibacterial Hand Wipes Spring Bliss by
Drug Labeling and Warnings
Wet Ones Antibacterial Hand Wipes Spring Bliss by is a Otc medication manufactured, distributed, or labeled by Edgewell Personal Care Brands LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
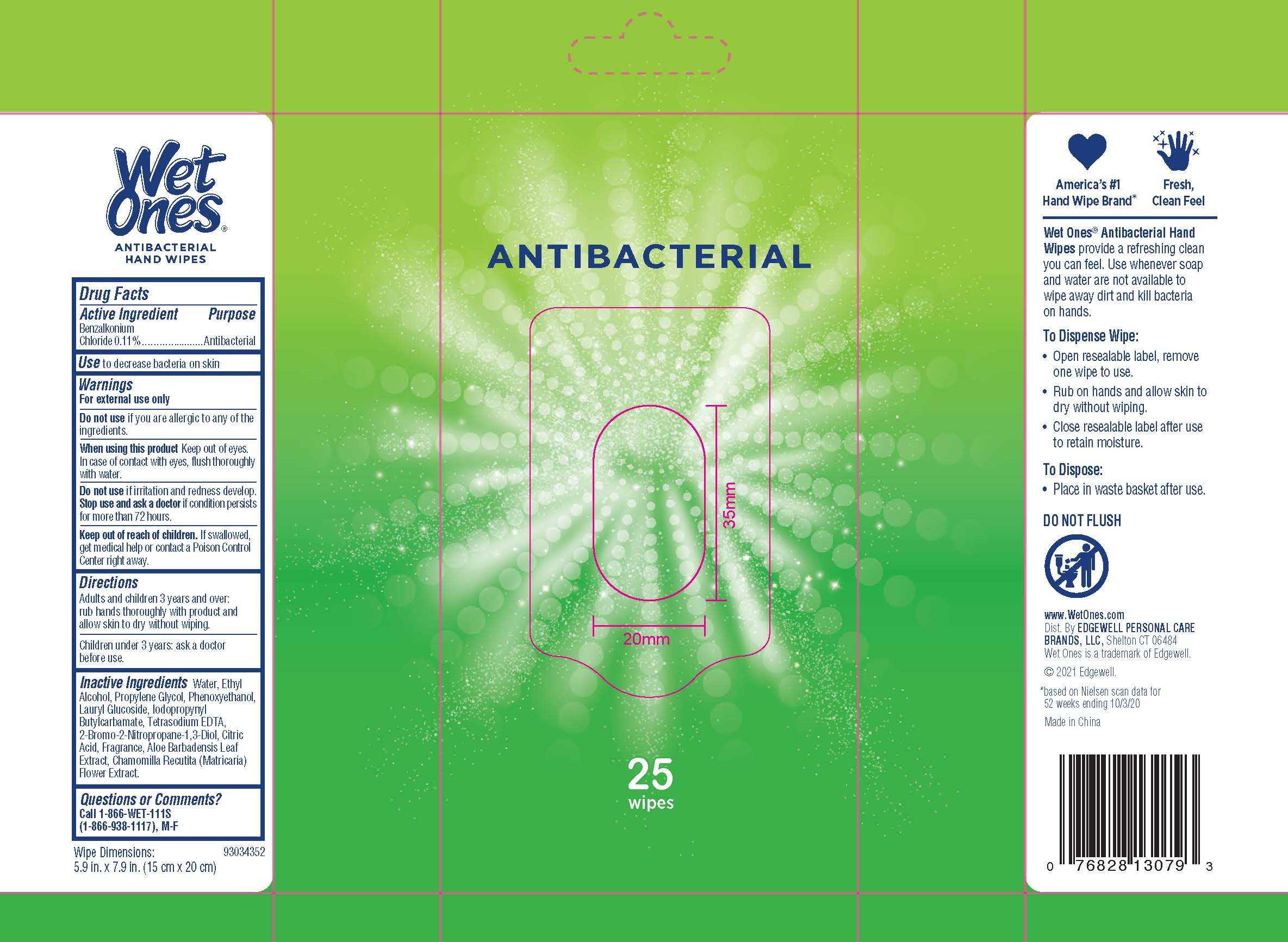
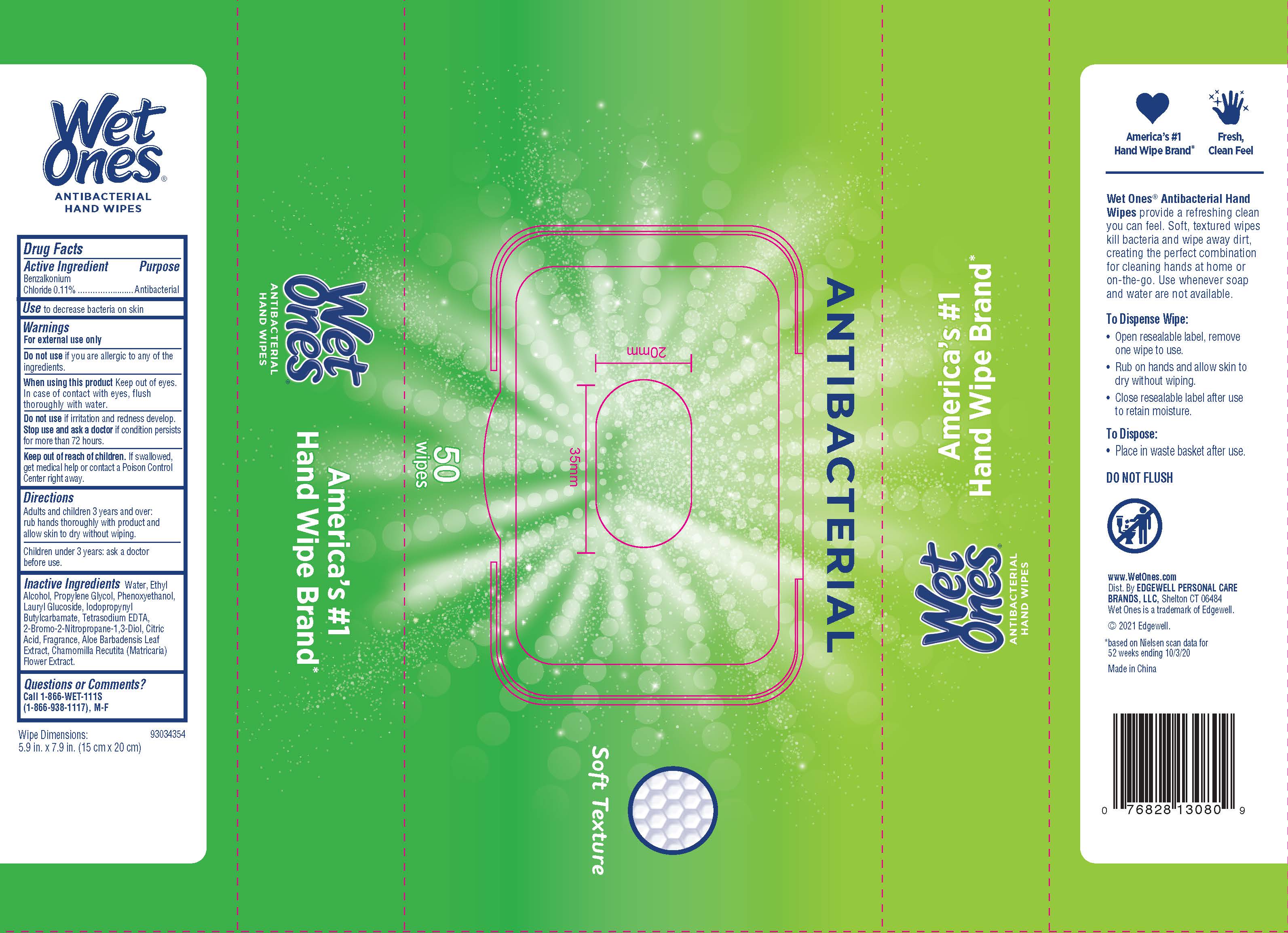
WET ONES ANTIBACTERIAL HAND WIPES SPRING BLISS- benzalkonium chloride swab
Edgewell Personal Care Brands LLC
----------
Warnings
For external use only
Directions
Adults and children 3 years and over: rub hands thoroughly with product and allow skin to dry without wiping
Children under 3 years: ask a doctor before use
| WET ONES ANTIBACTERIAL HAND WIPES SPRING BLISS
benzalkonium chloride swab |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Edgewell Personal Care Brands LLC (151179769) |
Revised: 10/2024
Document Id: 2371bff7-9ac2-6ede-e063-6394a90a6c6d
Set id: b848515f-fba0-21df-e053-2995a90a6ab5
Version: 2
Effective Time: 20241001