Clinol Sanitizing Hand Wipes 75% Alcohol
Clinol Sanitizing Hand Wipes 75% Alcohol by
Drug Labeling and Warnings
Clinol Sanitizing Hand Wipes 75% Alcohol by is a Otc medication manufactured, distributed, or labeled by Clinol Chemicals LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
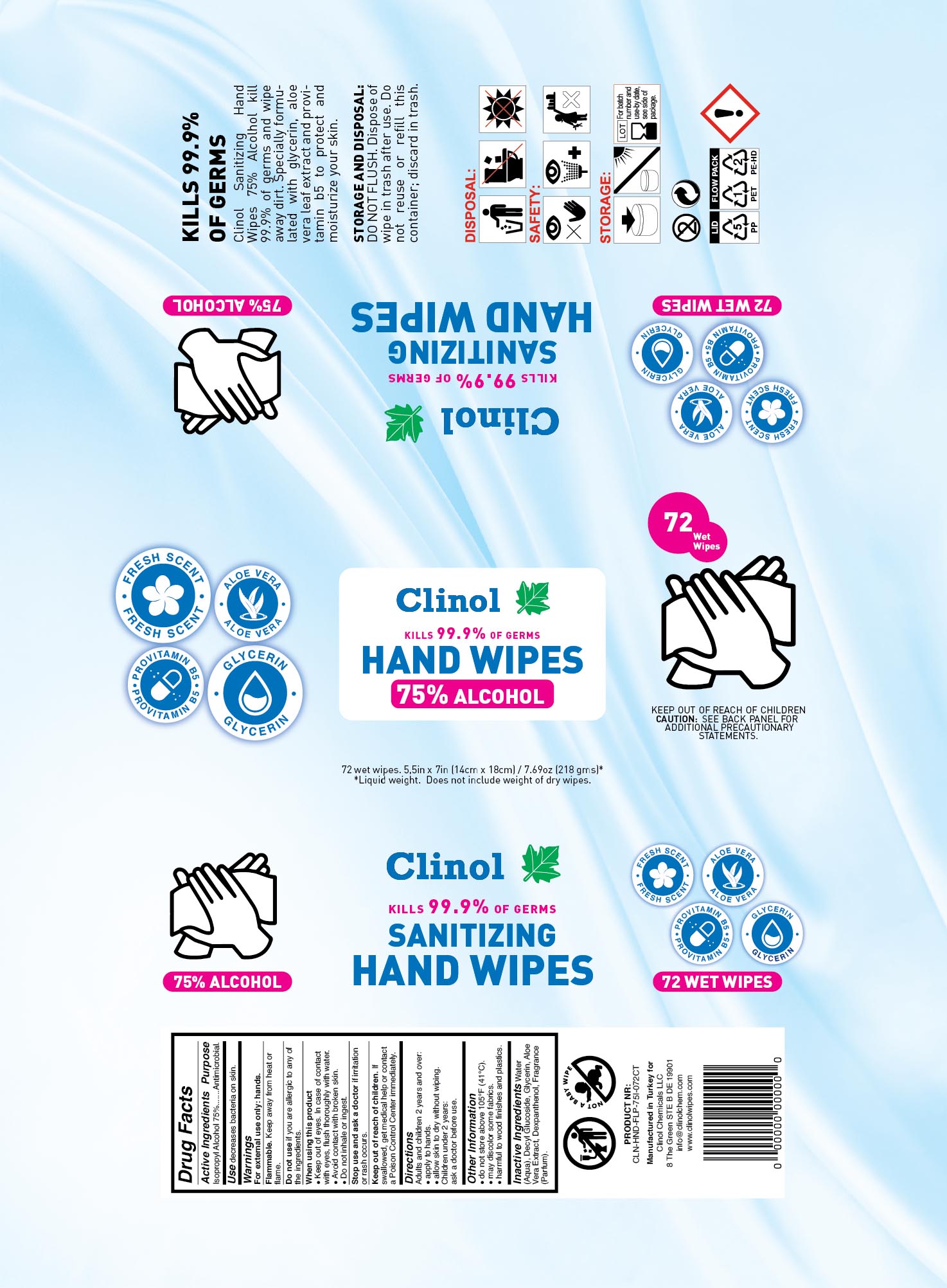
CLINOL SANITIZING HAND WIPES 75% ALCOHOL- isopropyl alcohol cloth swab
Clinol Chemicals LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Clinol Sanitizing Hand Wipes 75% Alcohol
When using this product
- Keep out of eyes. In case of contact with eyes, flush thoroughly with water.
- Avoid contact with broken skin.
- Do not inhale or ingest.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately
Directions
Adults and children 2 years and over :
- apply to hands.
- allow skin to dry without wipping.
Children under 2 years: ask a doctor before use.
Other information
- do not store above 105°F (41°C).
- may discolor some fabrics.
- harmful to wood finishes and plastics.
Inactive ingredients
Water (Aqua), Decyl Glucoside, Glycerin, Aloe Vera Extract, Dexpanthenol, Fragrance (Parfum).
Package Label - Principal Display Panel
Clinol Sanitizing Hand Wipes 75% Alcohol
Clinol
KILLS 99.9% OF GERMS
SANITIZING
HAND WIPES
75% ALCOHOL
FRESH SCENT
ALOE VERA
PROVITAMIN B5
GLYCERIN
Clinol 75% Alcohol Sanitizing Hand Wipes kill 99.9% of germs and wipe away dirt. Specially formulated with glycerin, aloe vera leaf extract and provitamin b5 to protect and moisturize your skin.
DIRECTIONS TO OPEN PACKAGE
Flip open dispensing cap. Remove and discard sealing foil. Locate wipe at center of roll and pull through small opening in lid. To avoid injury do not push finger through opening. For best results dispense wipes at an angle. Snap lid cap shut to retain moisture after use.
STORAGE AND DISPOSAL
DO NOT FLUSH. Dispose of wipe in trash after use. Do not reuse or refill this container; discard in trash.
KEEP OUT OF REACH OF CHILDREN
CAUTION: SEE BACK PANEL FOR ADDITIONAL PRECAUTIONARY STATEMENTS.
Manufactured in Turkey for
Clinol Chemicals LLC
8 The Green STE B DE 19901
info@clinolchem.com
www.clinolwipes.com
Packaging
Package size: 40 Wipes, NDC: 81370-002-01

Package size: 80 Wipes, NDC: 81370-002-02

Package size: 100 Wipes, NDC: 81370-002-03

Package size: 120 Wipes, NDC: 81370-002-04

Package size: 150 Wipes, NDC: 81370-002-05

Package size: 350 Wipes, NDC: 81370-002-06

Package size: 450 Wipes, NDC: 81370-002-07

Package size: 72 Wipes, NDC: 81370-002-08
| CLINOL SANITIZING HAND WIPES 75% ALCOHOL
isopropyl alcohol cloth swab |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Clinol Chemicals LLC (117779725) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.