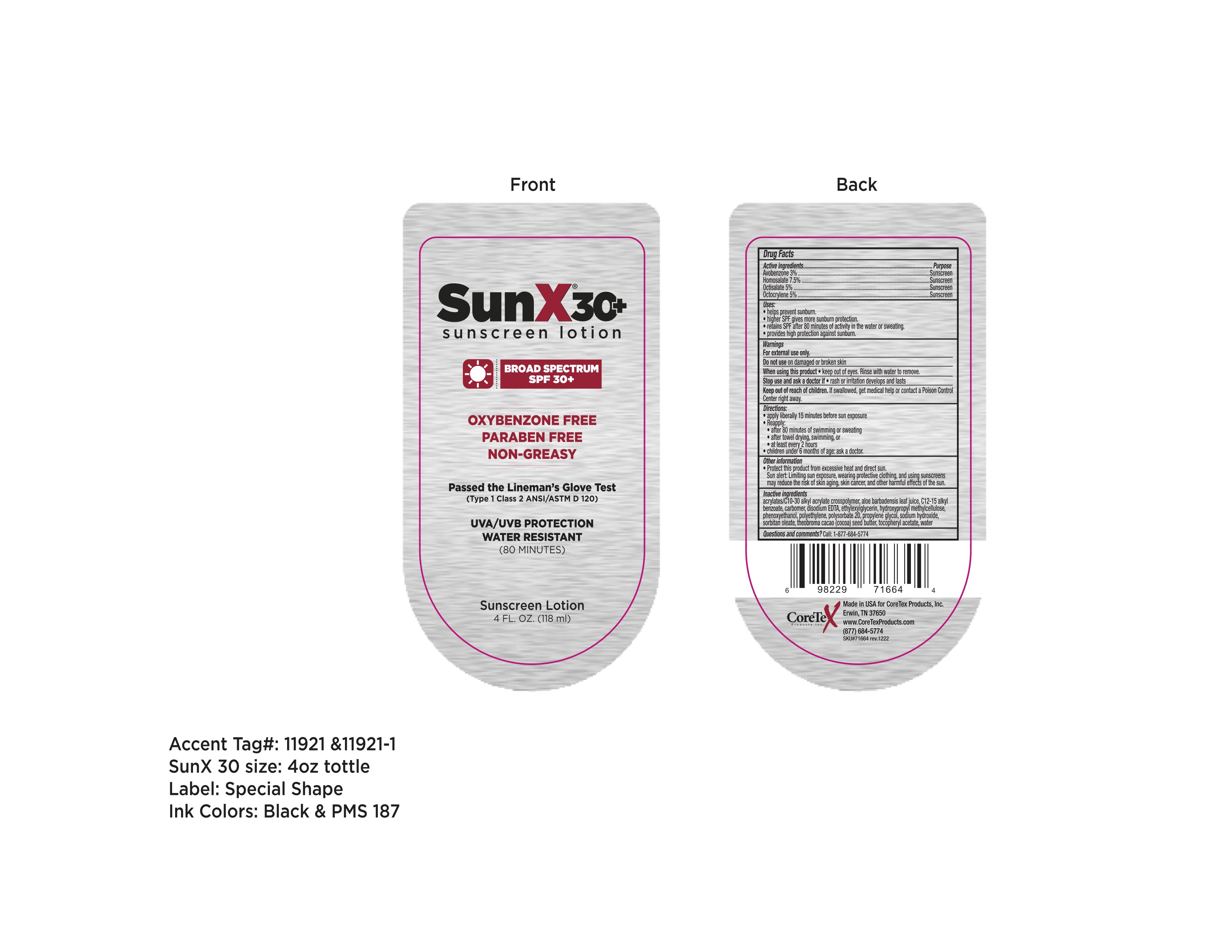
Sun X SPF 30 Thick New formulation 65753-106
CoreTex Sun X SPF 30 New by
Drug Labeling and Warnings
CoreTex Sun X SPF 30 New by is a Otc medication manufactured, distributed, or labeled by CoreTex Products Inc, Prime Enterprises. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CORETEX SUN X SPF 30 NEW- avobenzone, homosalate, octisalate, octocrylene lotion
CoreTex Products Inc
----------
Sun X SPF 30 Thick New formulation
65753-106
Uses
- helps prevent sunburn.
- higher SPF gives more sunburn protection.
- retains SPF after 80 minutes of activity in the water or sweating.
- provides high protection against sunburn.
Directions
- apply liberally 15 minutes before sun exposure
- Reapply:
- after 80 minutes of swimming or sweating
- after towel drying, swimming, or
- at least every 2 hours
- children under 6 months of age: ask a doctor.
Other information
- Protect this product from excessive heat or direct sun.
Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risk of skin aging, skin cancer, and other harmful effects of the sun.
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, aloe barbadensis leaf juice, C12-15 alkyl benzoate, carbomer, disodium EDTA, ethylhexylglycerin, hydroxypropyl methylcellulose, phenoxyethanol, polyethylene, polysorbate 20, propylene glycol, sodium hydroxide, sorbitan oleate, theobroma cacao (cocoa) seed butter, tocopheryl acetate, water.
| CORETEX SUN X SPF 30 NEW
avobenzone, homosalate, octisalate, octocrylene lotion |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - CoreTex Products Inc (061944620) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CoreTex Products Inc | 061944620 | pack(65753-106) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Prime Enterprises | 101946028 | manufacture(65753-106) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.