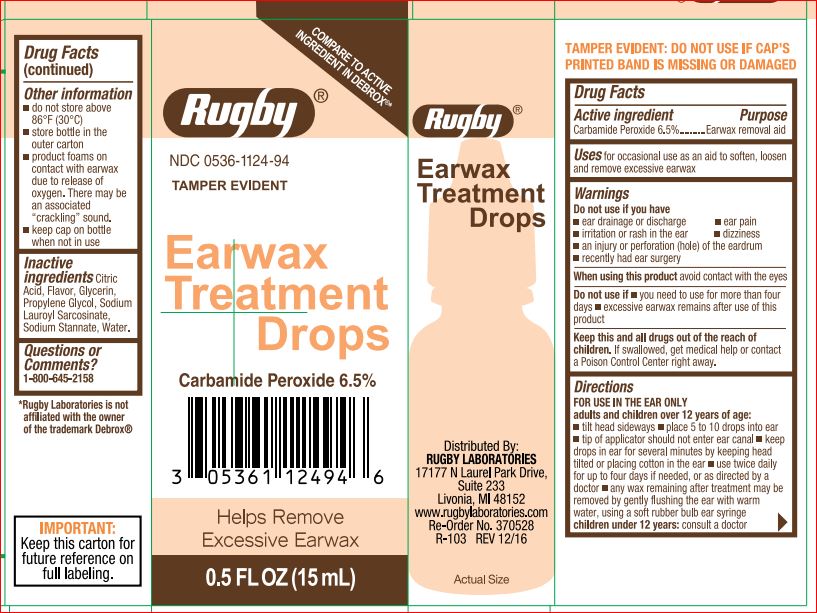
Earwax Treatment Drops by Rugby Laboratories / Product Quest Mfg. Drug Facts
Earwax Treatment Drops by
Drug Labeling and Warnings
Earwax Treatment Drops by is a Otc medication manufactured, distributed, or labeled by Rugby Laboratories, Product Quest Mfg.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
EARWAX TREATMENT DROPS RUGBY- carbamide peroxide 6.5% liquid
Rugby Laboratories
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Warnings
Ask doctor before use if you have ear drainage or discharge ear pain irritation or rash in ear dizziness an injury or perforation (hole) of the eardrum recently had ear surgery
DirectionsFor use in the ear only.
Adults and children over 12 years of age:
tilt head sideways and place 5 to 10 drops into ear
tip of applicator should not enter ear canal
keep drops in ear for several minutes by keeping head tilted or placing cotton in the ear
use twice daily for up to 4 days if needed, or as directed by a doctor
any wax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe
Children under 12 years of age: consult a doctor.
| EARWAX TREATMENT DROPS
RUGBY
carbamide peroxide 6.5% liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Rugby Laboratories (079246066) |
| Registrant - Product Quest Mfg. (927768135) |