ENDONESIC- menthol, camphor gel
ENDONESIC by
Drug Labeling and Warnings
ENDONESIC by is a Otc medication manufactured, distributed, or labeled by Blaine Labs Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- OTHER SAFETY INFORMATION
- ASK DOCTOR
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
INACTIVE INGREDIENTS: Ethyl Alcohol, Deionized Water, Glycerin,
Propylene Glycol, Cannabis Sativa Seed Oil (Hemp Seed Oil),
Cannabidiol (CBD), Arnica Montana Flower Extract (Arnica Oil),
Mentha Piperita (Peppermint) Oil, Mentha Viridis (Spearmint)
Leaf Oil, Camellia Sinensis Leaf Extract, Aloe Barbadensis Leaf
Extract, Hamamelis Virginiana (Witch Hazel) Extract, Melaleuca
Alternifolia (Tea Tree) Leaf Oil, Capsicum Annuum Fruit Extract,
Phenoxyethanol, Caprylyl Alcohol, Ethylhexyl Glycerin, Hexylene Glycol
-
Principal Display Panel
ENDONESIC ®
MAXIMUM
PAIN
RELIEFNDC# 63347-710-10
ENDONESIC®
1.7 FL OZ (50.275mL)
menthol and camphor
in a gel containing capsaicin and hemp oilPHYSICIAN FORMULATED PAIN-RELIEF GEL
MAXIMUM
PAIN
RELIEFGoes on cool and
progressively heatsInfused with Capsaicin,
Hemp Oil, and Tea Tree OilParaben-free formulation
Light formula absorbs into
the skin rapidlyLonger lasting formula. One
application offers relief for
up to 4-6 hoursProvides fast relief from sore
muscles, aches and painMADE IN USA
HEMP DERIVED
THC√Free
6 16728 0005 2
MANUFACTURED BY:
BLAINE LABS, INC
11037 LOCKPORT PLACE,
SANTA FE SPRINGS, CA 90670
www.BLAINELABS.com
PLVV-END-BX03 REV201 81 203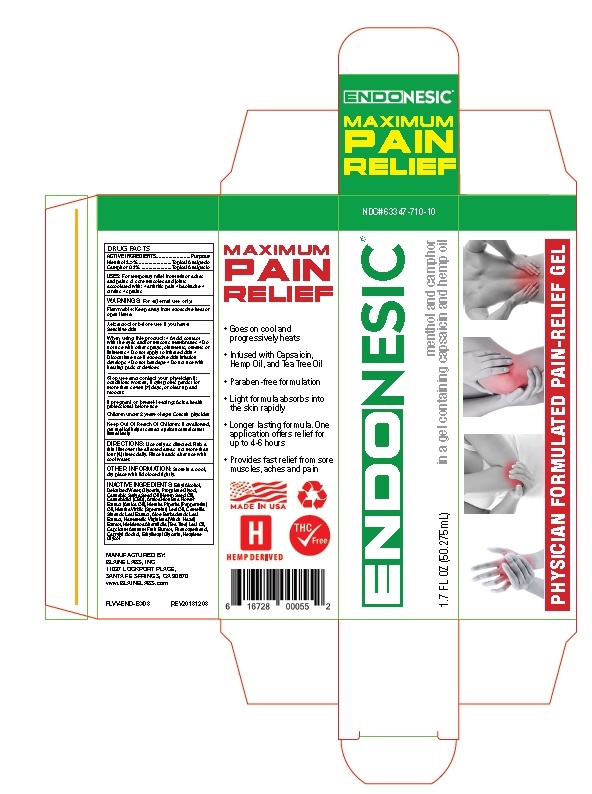
↓1.7 OZ Label↓

res
-
INGREDIENTS AND APPEARANCE
ENDONESIC
menthol, camphor gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63347-710 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1.7596 g in 50.275 mL CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 0.1006 g in 50.275 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) CANNABIDIOL (UNII: 19GBJ60SN5) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) PEPPERMINT OIL (UNII: AV092KU4JH) SPEARMINT OIL (UNII: C3M81465G5) GREEN TEA LEAF (UNII: W2ZU1RY8B0) ALOE VERA LEAF (UNII: ZY81Z83H0X) WITCH HAZEL (UNII: 101I4J0U34) TEA TREE OIL (UNII: VIF565UC2G) PAPRIKA (UNII: X72Z47861V) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLIC ALCOHOL (UNII: NV1779205D) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HEXYLENE GLYCOL (UNII: KEH0A3F75J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63347-710-10 1 in 1 BOX 12/01/2018 1 50.275 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 12/01/2018 Labeler - Blaine Labs Inc. (017314571) Establishment Name Address ID/FEI Business Operations Blaine Labs Inc. 017314571 manufacture(63347-710)
Trademark Results [ENDONESIC]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ENDONESIC 88214848 not registered Live/Pending |
Vivera Pharmaceuticals, Inc. 2018-12-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.