WINRHO SDF (rho(d) immune globulin intravenous- human injection
WinRho SDF by
Drug Labeling and Warnings
WinRho SDF by is a Prescription medication manufactured, distributed, or labeled by Saol Therapeutics Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use WinRho® SDF safely and effectively. See full prescribing information for WinRho® SDF.
WinRho® SDF [Rho(D) Immune Globulin Intravenous (Human)]
Solution for Intravenous or Intramuscular Injection
Initial U.S. Approval: 1995WARNING: INTRAVASCULAR HEMOLYSIS (IVH) IN IMMUNE THROMBOCYTOPENIC PURPURA (ITP)
See full prescribing information for complete boxed warning. This warning does not apply to Rho(D)-negative patients treated for the suppression of Rh isoimmunization.
- Intravascular hemolysis (IVH) leading to death has been reported in patients treated for ITP with WinRho SDF.
- IVH can lead to clinically compromising anemia and multi-system organ failure including acute respiratory distress syndrome (ARDS)
- Serious complications including severe anemia, acute renal insufficiency, renal failure and disseminated intravascular coagulation (DIC) have also been reported.
- Closely monitor patients treated with WinRho® SDF for ITP in a healthcare setting for at least 8 hours after administration. Perform dipstick urinalysis to monitor for hematuria and hemoglobinuria at baseline and 2 hours, 4 hours and prior to the end of the monitoring period. Alert patients and monitor the signs and symptoms of IVH including back pain, shaking chills, fever, and discolored urine or hematuria. Absence of these signs and/or symptoms of IVH within 8 hours do not indicate IVH cannot occur subsequently. Perform post-treatment laboratory tests if signs and/or symptoms of IVH are present or suspected after WinRho® SDF administration (5.2).
- If ITP patients are to be transfused after receiving WinRho® SDF, use Rho(D)-negative red blood cells (PRBCs) so as not to exacerbate ongoing hemolysis.
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
WinRho® SDF is a Rho(D) Immune Globulin Intravenous (Human) indicated for: Immune Thrombocytopenic Purpura (ITP) (1.1)
Raising platelet counts in Rho(D) positive, non-splenectomized:
- children with chronic or acute ITP
- adults with chronic ITP
- children and adults with ITP secondary to HIV infection Suppression of Rhesus (Rh) Isoimmunization (1.2)
- Pregnancy and other obstetric conditions in non-sensitized, Rho(D)-negative women with a Rh-incompatible pregnancy including:
- Routine antepartum and postpartum Rh prophylaxis
- Rh prophylaxis in obstetric complications or invasive procedures
- Incompatible transfusions in Rho(D)-negative individuals transfused with blood components containing Rho(D)-positive red blood cells (RBCs).
DOSAGE AND ADMINISTRATION
IU, International Units; mcg, micrograms
ITP (2.1) (IV administration only) Dose IU Dose mcg Rate of Administration 250 IU kg body weight 50 mcg/kg body weight Single injection over 3 to 5 minutes Suppression of Rh Isoimmunization (2.1) (IV or IM administration only) Indication Timing Dose (IV or IM) Dose (IV or IM) Pregnancy and other obstetric
conditionsIU
(international
units)mcg
(micrograms)Routine antepartum prophylaxis 28-weeks
gestation1,500 IU 300 mcg Postpartum prophylaxis Within 72 hours of delivery 600 IU 120 mcg Threatened abortion Immediately 1,500 IU300 mcg 300 mcg Amniocentesis and chorionic villus sampling before 34-weeks gestation Immediately after procedure 1,500 IU 300 mcg Abortion, amniocentesis, or other manipulation after 34-weeks gestation Within 72 hours 600 IU 120 mcg Incompatible transfusions or massive fetal hemorrhage Within 72 hours of exposure IV administration of 90 IU per 1 mL transfused RBC or per 2 mL transfused whole blood IV administration of 18 mcg per 1 mL transfused RBC or per 2 mL transfused whole blood DOSAGE FORMS AND STRENGTHS
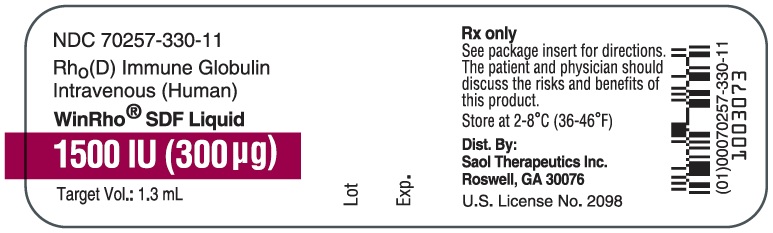
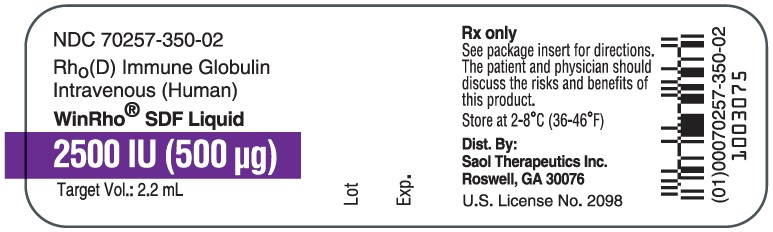
WinRho® SDF is a ready to use solution in single dose vials of 600 IU (120 mcg), 1,500 IU (300 mcg), 2,500 IU (500 mcg), 5,000 IU (1,000 mcg) and 15,000 IU (3,000 mcg).
CONTRAINDICATIONS
- Patients with known anaphylactic or severe hypersensitivity responses to human immune globulin products.
- IgA-deficient patients with antibodies to IgA or a history of hypersensitivity reaction to WinRho® SDF or any of its components.
- Patients with a history of autoimmune hemolytic anemia, with pre existing hemolysis or at high risk for hemolysis.
- Infants for the suppression of Rho(D) isoimmunization.
WARNINGS AND PRECAUTIONS
- Hypersensitivity: severe, including anaphylaxis (5.1)
- Intravascular hemolysis (IVH) with ITP treatment: hemolysis (5.3). hemolytic anemia and IVH complications (5.2). Obtain baseline labs (5.9).
- Transmissible infectious agents: e.g., viruses and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent (5.4).
- Acute renal dysfunction/failure (5.5): monitor labs of those at risk (5.9).
- Thrombotic events: consider blood viscosity labs for those at risk (5.6)
- Passive transfer of antibodies may confound serologic testing. (5.7).
- Transfusion-related acute lung injury [TRALI]) (5.8): monitor for respiratory adverse events and, if they occur, test for anti-neutrophil antibodies (5.9).
- Blood glucose test monitoring interference (5.10).
ADVERSE REACTIONS
The most common adverse reactions occurring in ≤ 2% of doses are headache, chills, fever, asthenia, pallor, diarrhea, nausea, vomiting, arthralgia, myalgia, dizziness, malaise, hyperkinesia, abdominal or back pain, hypotension, hypertension, increased LDH, somnolence, vasodilation, pruritus, rash and sweating. (6.1)
Serious adverse reactions, such as IVH, clinically compromising anemia, acute renal insufficiency and DIC have been observed in patients receiving WinRho® SDF for treatment of ITP. Some of these cases resulted in fatal outcome. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Saol Therapeutics Inc. at 1-833-644-4216 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch..
DRUG INTERACTIONS
Immunoglobulin administration may transiently interfere with the immune response to live virus vaccines, such as measles, mumps and rubella. (7.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: INTRAVASCULAR HEMOLYSIS (IVH)
1 INDICATIONS AND USAGE
1.1 Treatment of ITP
1.2 Suppression of Rh Isoimmunization
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Intravascular Hemolysis (IVH) for ITP Treatment
5.3 Hemolysis for ITP Treatment
5.4 Transmissible Infectious Agents
5.5 Acute Renal Insufficiency/Failure
5.6 Thromboembolic Events
5.7 Interference with Serological Testing
5.8 Transfusion-Related Acute Lung Injury (TRALI)
5.9 Monitoring: Laboratory Tests
5.10 Interference with Blood Glucose Testing: False High Blood Glucose Levels
5.11 Suppression of Rh Isoimmunization
6 ADVERSE REACTIONS
6.1 Clinical Trials Experiences
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
7.1 Live Virus Vaccines
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
14 CLINICAL STUDIES
14.1 Treatment of ITP
14.2 Suppression of Rh Isoimmunization
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 FDA Approved Patient Labeling – (Patient Prescribing Information)
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: INTRAVASCULAR HEMOLYSIS (IVH)
This warning does not apply to Rho(D)-negative patients treated for the suppression of Rh isoimmunization.
- Intravascular hemolysis (IVH) leading to death has been reported in patients treated with WinRho® SDF for immune thrombocytopenic purpura (ITP).
- IVH can lead to clinically compromising anemia and multi-system organ failure including acute respiratory distress syndrome (ARDS).
- Serious complications including severe anemia, acute renal insufficiency, renal failure and disseminated intravascular coagulation (DIC) have also been reported.
- Closely monitor patients treated with WinRho® SDF for ITP in a healthcare setting for at least 8 hours after administration. A dipstick urinalysis to monitor for hematuria and hemoglobinuria is to be performed at baseline and then after administration at 2 hours, 4 hours and prior to the end of the monitoring period. Alert patients and monitor the signs and symptoms of IVH including back pain, shaking chills, fever, and discolored urine or hemoglobinuria. Absence of these signs and/or symptoms of IVH within 8 hours do not indicate IVH cannot occur subsequently. If signs and/or symptoms of IVH are present or suspected after WinRho® SDF administration, post-treatment laboratory tests should be performed including plasma hemoglobin, haptoglobin, LDH, and plasma bilirubin (direct and indirect).
- If ITP patients are to be transfused after receiving WinRho® SDF, use Rho(D)-negative red blood cells (PRBCs) so as not to exacerbate ongoing hemolysis.
-
1 INDICATIONS AND USAGE
WinRho® SDF is a Rho(D) Immune Globulin Intravenous (Human) (anti-D) product that is indicated for the treatment of ITP in Rho(D)-positive patients and for the suppression of Rh isoimmunization in non-sensitized Rho(D)-negative patients.
1.1 Treatment of ITP
WinRho® SDF is indicated for use in clinical situations requiring an increase in platelet count to prevent excessive hemorrhage in the treatment of non-splenectomized, Rho(D)-positive
- children with chronic or acute ITP
- adults with chronic ITP
- children and adults with ITP secondary to HIV infection
The safety and efficacy of WinRho® SDF have not been evaluated in clinical trials for patients with non-ITP causes of thrombocytopenia or in previously splenectomized patients or in patients who are Rho(D)-negative.
1.2 Suppression of Rh Isoimmunization
Pregnancy and Other Obstetric Conditions
WinRho® SDF is indicated for the suppression of Rh isoimmunization in non-sensitized, Rho(D)-negative (D-negative) women with a Rh-incompatible pregnancy, including:
- Routine antepartum and postpartum Rh prophylaxis
- Rh prophylaxis in cases of:
- – Obstetric complication (e.g., miscarriage, abortion, threatened abortion, ectopic pregnancy or hydatidiform mole, transplacental hemorrhage resulting from antepartum hemorrhage)
- – Invasive procedures during pregnancy (e.g., amniocentesis, chorionic biopsy) or obstetric manipulative procedures (e.g., external version, abdominal trauma)
A Rh-incompatible pregnancy is assumed if the fetus/baby is either Rho(D)-positive or Rho(D)-unknown or if the father is either Rho(D)-positive or Rho(D)-unknown.
Incompatible Transfusions
WinRho® SDF is indicated for the suppression of Rh isoimmunization in Rho(D)-negative individuals transfused with Rho(D)-positive red blood cells (RBCs) or blood components containing Rho(D)-positive RBCs.
WinRho® SDF is not indicated for use as immunoglobulin replacement therapy for immune globulin deficiency syndromes.
-
2 DOSAGE AND ADMINISTRATION
For intravenous or intramuscular use only.
2.1 Dose
Treatment of ITP
ADMINISTER WinRho® SDF BY THE INTRAVENOUS ROUTE ONLY.
Proper care should be taken when calculating the dose of WinRho® SDF to be administered. A confusion between International Units (IU) and micrograms (mcg) of product (1 mcg = 5 IU), could result in either an overdose that could lead to a severe hemolytic reaction or a dose too low to be effective.
Since Win Rho® SDF is administered on a weight-based regimen per kilogram (kg), patient weight determination must be taken in kilograms (kg) as inappropriate use of pounds (lbs) will result in significant overdosing of WinRho® SDF.
Please note that dose for WinRho® SDF may be calculated using either international units (IU) or micrograms (mcg) per kilograms.
Table 1 provides dosing guidelines for ITP patients.
Table 1: ITP Dosing Guidelines (Intravenous use only) - All patients should be monitored to determine clinical response by assessing platelet counts, RBCs, hemoglobin (Hgb), and reticulocyte levels [see Warnings and Precautions (5.2)]
- Safety and efficacy of WinRho® SDF® in the treatment of ITP at doses exceeding 300 IU/kg (60 mcg/kg) has not been established.
- Treatment is rarely indicated in patients with platelet count above 50x109/L.
- To determine the dosage and number of vials needed for the treatment of ITP:
weight in lbs/2.21 = weight in kg
weight in kg X selected IU (mcg) dosing level = dosage
dosage / vial size = number of vials needed
Indication Initial Dose
(dose in either IU or mcg)Subsequent Doses
(dose in either IU or mcg)ITP
(intravenous use only)Single intravenous dose
or
divided doses given on two separate daysDetermine frequency by clinical response (platelet counts, RBC, Hgb and reticulocyte count) Hemoglobin ≥ 10 g/dl 250 IU/kg 50 mcg/kg 250-300 IU/kg 50-60 mcg/kg Hemoglobin 8 to <10 g/dl
(use alternative treatments
for Hgb <8 g/dl)125-200 IU/kg 25-40 mcg/kg 125-200 IU/kg 25-40 mcg/kg Suppression of Rh Isoimmunization
Table 2: Rh Isoimmunization Dosing Guidelines - If the Rh status of the baby is not known at 72 hours, administer WinRho® SDF to the mother at 72 hours after delivery. If more than 72 hours have elapsed, administer as soon as possible up to 28 days after delivery.
- If WinRho® SDF is administered early in pregnancy for amniocentesis and chorionic villus sampling (before 34 weeks), then administer WinRho® SDF repeatedly at 12-week interval to maintain adequate levels of passively acquired anti-Rh.
Indication Initial Dose
(dose in either IU or mcg)Subsequent Doses
(dose in either IU or mcg)Suppression of Rh Isoimmunization
(intravenous or intramuscular)Single intravenous or
intramuscular doseRoutine antepartum prophylaxis in Rh-
incompatible pregnancy (28 weeks gestation)1,500 IU 300 mcg Postpartum
(within 72 hours of birth of Rh0(D) positive
newborn)600 IU 120 mcg Threatened abortion (immediately) 1,500 IU 300 mcg Amniocentesis and chorionic villus sampling
before 34 weeks gestation (immediately
following procedure, then every 12 weeks
during pregnancy)1,500 IU 300 mcg 1,500 IU
(every 12
weeks)300 mcg
(every 12
weeks)Abortion, amniocentesis, or any other
manipulation after 34 weeks gestation (within
72 hours)600 IU 120 mcg Incompatible Transfusion
Table 3: Incompatible Transfusion Dosing Guidelines Indication Dose
(dose in either IU or mcg)Frequency Incompatible Transfusion
(intravenous or intramuscular)Begin treatment within 72 hours of exposure Intravenous If exposed to Rh0(D)-Positive
Whole Blood45 IU per ml
blood9 mcg per ml blood Every 8 hours If exposed to Rh0(D)-Positive
Red Blood Cells90 IU per ml
cells18 mcg per ml cells Administer this dose until total dose has been administered 3000 IU 600 mcg Every 8 hours Intramuscular If exposed to Rh0(D)-Positive
Whole Blood60 IU per ml blood 12 mcg per ml
bloodEvery 12 hours If exposed to Rh0(D)-Positive
Red Blood Cells120 IU per ml cells 24 mcg per ml cells Administer this dose until total dose has been administered 6000 IU 1,200 mcg Every 12 hours For incompatible transfusion or massive fetal hemorrhage, treatment must occur within 72 hours and dose may be intravenous or intramuscular, based on the volume of blood or cells replaced.
2.2 Preparation
- Bring WinRho® SDF to room temperature prior to use.
- Inspect WinRho® SDF for particulate matter and discoloration prior to administration. Do not use if the solution is cloudy or contains particulates.
- WinRho® SDF is for single use only. Discard any unused portion.
- The solution is ready to use, no reconstitution required.
- See Table 4 for the target fill volumes for each of the dosage sizes for WinRho® SDF.
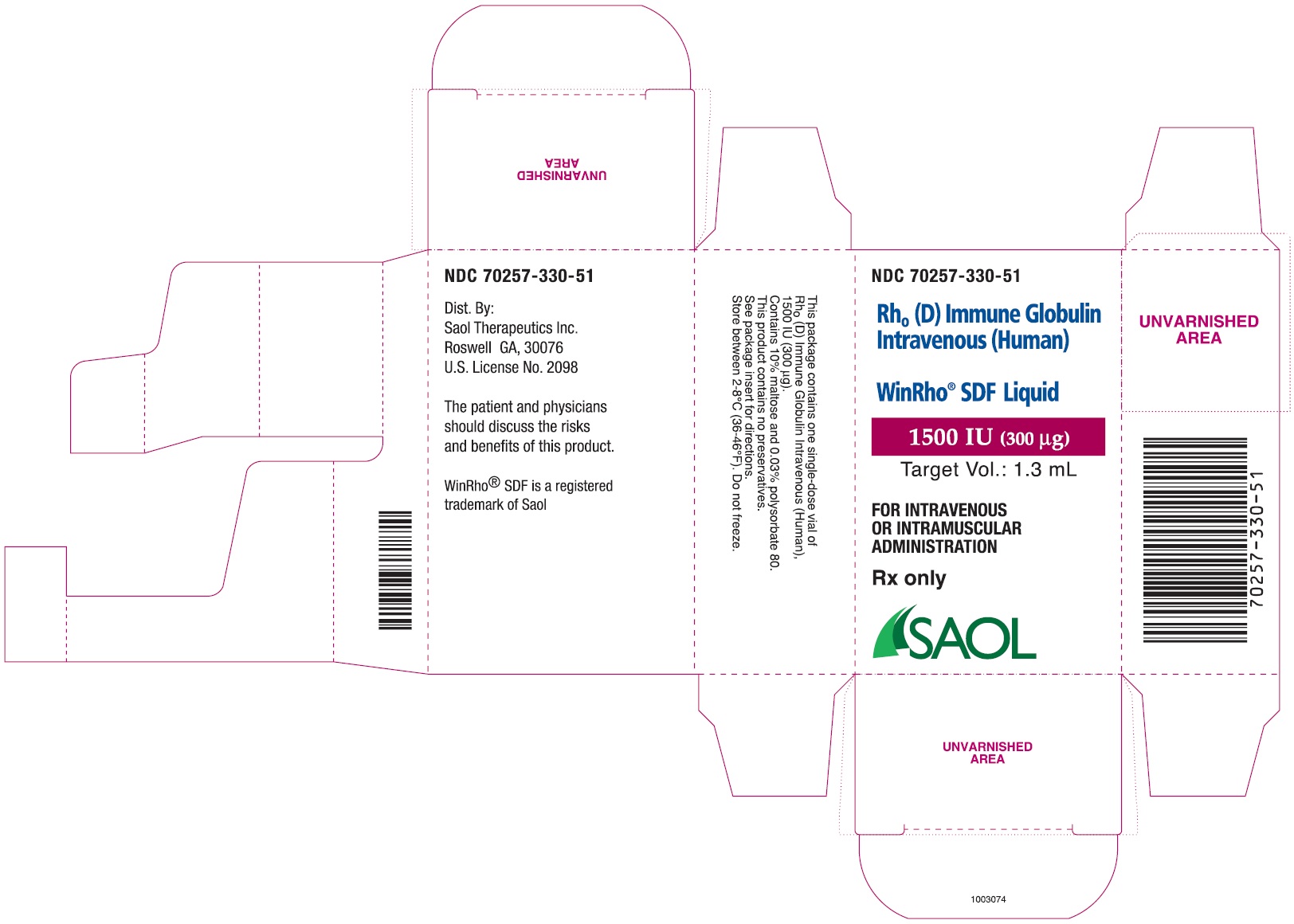
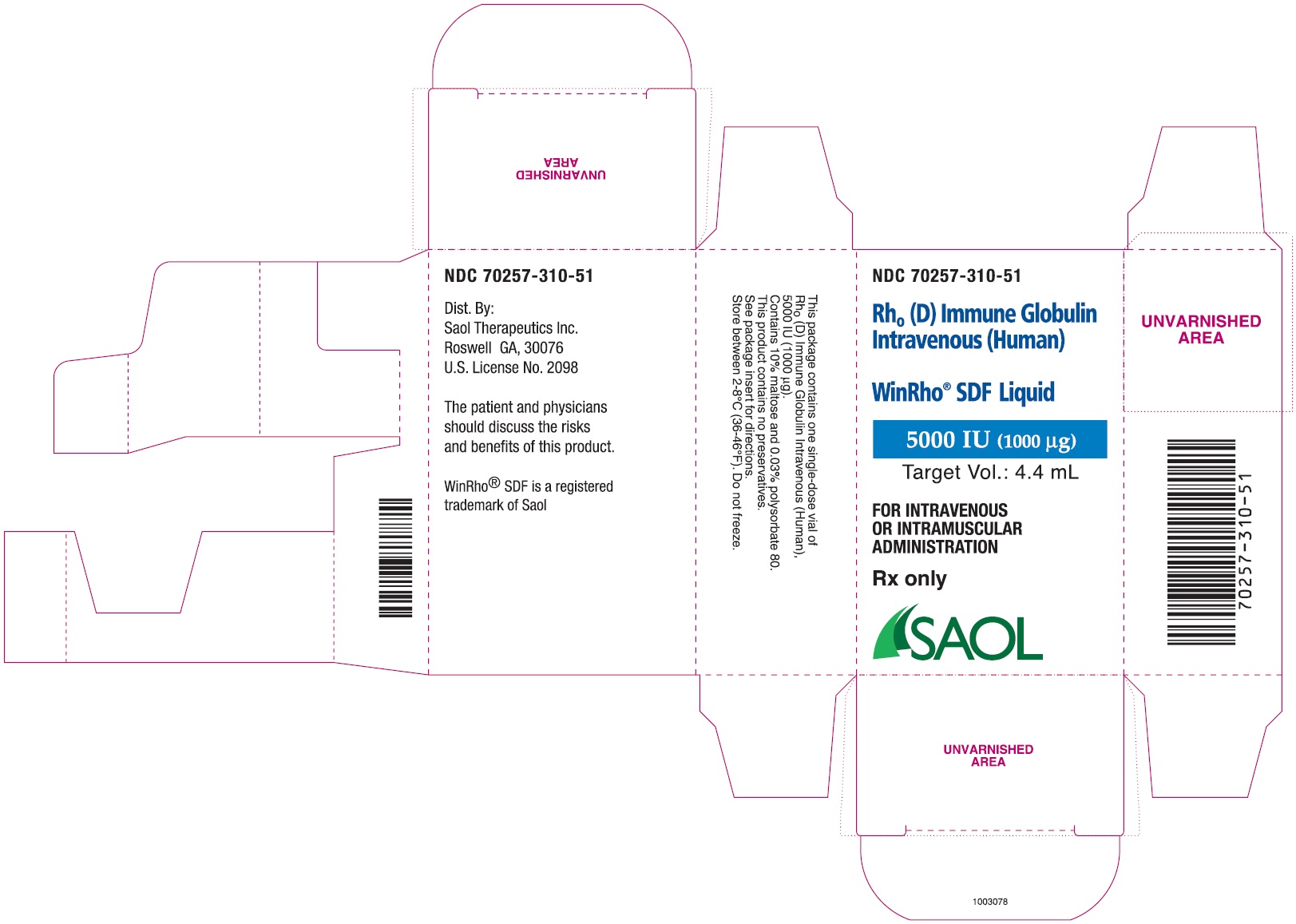
Table 4: Liquid WinRho® SDF Dosage Size and Target Fill Volumes Dose (IU) Dose (mcg) Target Fill Volume 600 IU 120 mcg 0.5 mL 1,500 IU 300 mcg 1.3 mL 2,500 IU 500 mcg 2.2 mL 5,000 IU 1,000 mcg 4.4 mL 15,000 IU 3,000 mcg 13.0 mL Note: Remove the entire contents of the vial to obtain the labelled dosage of WinRho® SDF. If partial vials are required for dosage calculation, withdraw the entire contents of the vial to ensure accurate calculation of the dosage requirement. For ease in withdrawing the contents of the vial, draw back the plunger of a sterile syringe (with the needle and needle cover in place) to admit air into the syringe. Depress the plunger of the syringe to inject air into the vial. Invert vial and aspirate contents of vial into syringe.
2.3 Administration
Route of administration depends on the indication as follows:
ITP Intravenous (IV) only Suppression of Rh Intravenous (IV) or intramuscular (IM) Incompatible transfusion Intravenous (IV) or intramuscular (IM) ITP
- Administer the entire dose of WinRho® SDF into a suitable vein over three to five minutes.
- Administer WinRho® SDF separately from other drugs.
- If dilution of WinRho® SDF is preferred prior to intravenous administration, use only normal saline as diluent. Do not use Dextrose (5%) in water (D5W).
Suppression of Rh Isoimmunization
- For intravenous administration, administer WinRho® SDF separately from other drugs. WinRho® SDF should be administered at a rate of 2 mL per 5 to 15 seconds.
- For intramuscular administration, administer into the deltoid muscle of the upper arm or the anterolateral aspects of the upper thigh. Due to the risk of sciatic nerve injury, avoid the gluteal region. If the gluteal region is used, use only the upper, outer quadrant.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
WinRho® SDF is contraindicated in:
- Patients who have had known anaphylactic or severe systemic reaction to the administration of human immune globulin products.
- IgA-deficient patients with antibodies to IgA or a history of hypersensitivity reaction to WinRho® SDF or any of its components.
- Patients with autoimmune hemolytic anemia, with pre-existing hemolysis or at high risk for hemolysis.
- Infants for the suppression of Rho(D) isoimmunization.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Severe hypersensitivity reactions may occur [see Contraindications (4)]. If symptoms of allergic or early signs of hypersensitivity reactions (including generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis) occur, discontinue WinRho® SDF infusion immediately and institute appropriate treatment. WinRho® SDF should be administered in a setting where appropriate equipment, medication such as epinephrine, and personnel trained in the management of hypersensitivity, anaphylaxis and shock are available.
WinRho® SDF contains ≤ 40 mcg/mL IgA [see Description (11)]. Patients with antibodies to IgA have a greater risk of developing potentially severe hypersensitivity and anaphylactic reactions. WinRho® SDF is contraindicated in IgA-deficient patients with antibodies to IgA or a history of hypersensitivity reaction to WinRho® SDF or any of its components [see Contraindications (4)].
WinRho® SDF contains 10% maltose, a disaccharide sugar derived from corn. Patients with corn allergy should avoid using WinRho® SDF due to risk of hypersensitivity. [see Contraindications (4)]
5.2 Intravascular Hemolysis (IVH) for ITP Treatment
IVH leading to death has been reported in patients treated for ITP with WinRho® SDF.
IVH can lead to clinically compromising anemia and multi-system organ failure including acute respiratory distress syndrome (ARDS).
Serious complications including severe anemia, acute renal insufficiency, renal failure and disseminated intravascular coagulation (DIC) have also been reported.7,8
Closely monitor patients treated with WinRho® SDF for ITP in a healthcare setting for at least 8 hours after administration. Perform a dipstick urinalysis to monitor for hematuria and hemoglobinuria at baseline and then after administration at 2 hours, 4 hours and prior to the end of the monitoring period. Alert patients and monitor for signs and symptoms of IVH including back pain, shaking chills, fever, and discolored urine or hemoglobinuria. Absence of these signs and/or symptoms of IVH within eight hours do not indicate IVH cannot occur subsequently. If signs and/or symptoms of IVH are present or if IVH is suspected after WinRho® SDF administration, perform post-treatment laboratory tests including plasma hemoglobin, haptoglobin, LDH, and plasma bilirubin (direct and indirect).
5.3 Hemolysis for ITP Treatment
Although the mechanism of action of WinRho® SDF in the treatment of ITP is not completely understood it is postulated that anti-D binds to the Rho(D) RBC resulting in formation of antibody-coated RBC complexes. Immune-mediated clearance of the antibody-coated RBC complexes would spare the antibody-coated platelets because of the preferential destruction of antibody-coated RBC complexes by the macrophages located in the reticuloendothelial system.9-11 The side effect of this action is a decrease in hemoglobin levels (extravascular hemolysis).7 The pooled data from ITP clinical studies demonstrated a mean decrease from baseline in hemoglobin levels of 1.2 g/dL within 7 days after administration of WinRho® SDF.
In patients with pre-disposing conditions, renal and cardiovascular complications of IVH may occur more frequently. Patients of advanced age (age over 65 years) with co-morbid conditions may be at an increased risk of developing sequelae from acute hemolytic reactions. If a patient has evidence of hemolysis (reticulocytosis greater than 3%) or is at high risk for hemolysis [positive direct antiglobulin test (DAT) not attributed to previous immune globulin administration], alternate therapies must be used.
If the patient has lower than normal hemoglobin levels (less than 10 g/dL), a reduced dose of 125 to 200 IU/kg (25 to 40 mcg/kg) should be given to minimize the risk of increasing the severity of anemia in the patient. Alternative treatments should be used in patients with hemoglobin levels that are less than 8 g/dL due to the risk of increasing the severity of the anemia [see Dose (2.1)].
Significant anemia may present with pallor, hypotension, or tachycardia while acute renal insufficiency may present with oliguria or anuria, edema and dyspnea. Patients with IVH who develop DIC may exhibit signs and symptoms of increased bruising and prolongation of bleeding time and clotting time which may be difficult to detect in the ITP population. Consequently, the diagnosis of this serious complication of IVH is dependent on laboratory testing [see Warnings and Precautions (5.9)]. Previous uneventful administration of WinRho® SDF does not preclude the possibility of an occurrence of IVH and its complications following any subsequent administration of WinRho® SDF. Have confirmatory laboratory testing on ITP patients presenting with signs and/or symptoms of IVH and its complications after anti-D administration [see Warnings and Precautions (5.9)]
If ITP patients are to be transfused, use Rho(D)-negative red blood cells (PRBCs) so as not to exacerbate ongoing hemolysis.
5.4 Transmissible Infectious Agents
Because WinRho® SDF is made from human plasma; it may carry a risk of transmitting infectious agents, e.g., viruses and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. The risk of transmitting an infectious agent has been reduced by screening plasma donors for prior exposure to certain pathogens, testing for the presence of certain current viral infections, and including virus inactivation/removal steps in the manufacturing process [see Description (11)].
Report all infections thought to have been transmitted by WinRho® SDF to Saol Therapeutics Inc. at 1-833-644-4216. The physician should discuss the risks and benefits of this product with the patient.
5.5 Acute Renal Insufficiency/Failure
Acute renal insufficiency/failure, osmotic nephropathy, acute tubular necrosis, proximal tubular nephropathy, and death may occur upon use of Immune Globulin Intravenous (IGIV) products, including WinRho® SDF.2 Ensure that patients are not volume depleted before administering WinRho® SDF. For patients at risk of renal insufficiency or failure, including those with any degree of pre-existing renal insufficiency, diabetes mellitus, advanced age (above 65 years of age), volume depletion, sepsis, paraproteinemia, or receiving known nephrotoxic drugs, administer WinRho® SDF at the minimum infusion rate practicable and assess renal function, including measurement of blood urea nitrogen (BUN) and serum creatinine, before the initial infusion of WinRho® SDF and at appropriate intervals thereafter.
5.6 Thromboembolic Events
Thromboembolic events may occur during or following treatment with WinRho® SDF and other IGIV products.3,4 Patients at risk include those with a history of atherosclerosis, multiple cardiovascular risk factors, advanced age, impaired cardiac output, coagulation disorders, prolonged periods of immobilization, history of arterial or venous thrombosis, estrogen use, indwelling central vascular catheters, and/or known/suspected hyperviscosity. Thrombosis may occur in the absence of known risk factors.
Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies. For patients who are at risk of developing thromboembolic events, administer WinRho® SDF at the minimum rate of infusion practicable.
5.7 Interference with Serological Testing
After administration of WinRho® SDF, a transitory increase of various passively transferred antibodies in the patient’s blood may yield positive serological testing results, with the potential for misleading interpretation. Passive transmission of antibodies to erythrocyte antigens (e.g., A, B, C and E) and other blood group antibodies [for example, anti Duffy, anti Kidd (anti JKa) antibodies]5 may cause a positive direct or indirect (Coombs’) test.
A large fetomaternal hemorrhage late in pregnancy or following delivery may cause a weak mixed field positive Du test result. Assess such an individual for a large fetomaternal hemorrhage and adjust the dose of WinRho® SDF accordingly. The presence of passively administered anti Rho(D) in maternal or fetal blood can lead to a positive direct Coombs’ test. If there is an uncertainty about the father’s Rh group or immune status, administer WinRho® SDF to the mother.
5.8 Transfusion-Related Acute Lung Injury (TRALI)
Non-cardiogenic pulmonary edema may occur in patients following IGIV treatment, including WinRho® SDF.6 TRALI is characterized by severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, and fever. Symptoms typically appear within 1 to 6 hours following administration of blood products.
Monitor patients for pulmonary adverse reaction. If TRALI is suspected, perform appropriate tests for the presence of anti-neutrophil antibodies and anti-HLA antibodies in both the product and patient serum. TRALI may be managed using oxygen therapy with adequate ventilatory support.
5.9 Monitoring: Laboratory Tests
- For all ITP patients, blood type, blood count, reticulocyte count, DAT and dipstick urinalysis are recommended before deciding to treat patients with WinRho® SDF. In patients with evidence of hemolysis (reticulocytosis greater than 3%), or patients at risk of hemolysis (positive DAT not attributed to previous immune globulin administration) use other treatments.1
- Closely monitor patients administered WinRho® SDF for at least 8 hours post administration and perform a dipstick urinalysis to monitor for hematuria and hemoglobinuria at baseline and then after administration at 2 hours, 4 hours and prior to the end of the monitoring period.
- If signs and/or symptoms of IVH and its complications are present after anti-D administration, perform appropriate confirmatory laboratory testing including, but not limited to, CBC (i.e. hemoglobin, platelet counts), haptoglobin, plasma hemoglobin, urine dipstick, assessment of renal function (i.e. BUN, serum creatinine), liver function (i.e. LDH, direct and indirect bilirubin) and DIC specific tests such as D-dimer or Fibrin Degradation Products (FDP) or Fibrin Split Products (FSP).
- Periodic monitoring of renal function and urine output in patients who are at increased risk of developing acute renal failure [see Warnings and Precautions (5.5)]. Assess renal function in these at-risk patients, including measurement of BUN and serum creatinine, before the initial infusion of WinRho® SDF and at appropriate intervals thereafter.
- If TRALI is suspected in ITP patients, perform appropriate tests for the presence of anti-neutrophil antibodies in both the product and patient serum [see Warnings and Precautions (5.9)].
5.10 Interference with Blood Glucose Testing: False High Blood Glucose Levels
The liquid formulation of WinRho® SDF contains maltose. Maltose in IGIV products has been shown to give falsely high blood glucose levels in certain types of blood glucose testing systems [for example, by systems based on glucose dehydrogenase pyrroloquinolinequinone (GDH-PQQ) or glucose-dye-oxidoreductase methods]. Due to the potential for falsely elevated glucose readings, only use testing systems that are glucose-specific to test or monitor blood glucose levels in patients receiving maltose-containing parenteral products, including WinRho® SDF Liquid.
Carefully review the product information of the blood glucose testing system, including that of the test strips, to determine if the system is appropriate for use with maltose-containing parenteral products. If any uncertainty exists, contact the manufacturer of the testing system to determine if the system is appropriate for use with maltose-containing parenteral products.
5.11 Suppression of Rh Isoimmunization
Do not administer WinRho® SDF to Rho(D)-negative individuals who are Rh immunized as evidenced by an indirect antiglobulin (Coombs’) test revealing the presence of anti-Rho(D) (anti-D) antibody. For postpartum use following an Rh-incompatible pregnancy administer WinRho® SDF to the mother only. Do not administer to the newborn infant.
-
6 ADVERSE REACTIONS
Serious adverse reactions, some of these cases resulted in fatal outcome, have been observed in patients receiving WinRho® SDF for the treatment of ITP. These include: intravascular hemolysis (IVH), clinically compromising anemia, acute renal insufficiency and DIC [see Adverse Reactions, (6.2)].
The most common adverse reactions observed for all indications are: headache, chills, fever, asthenia, pallor, diarrhea, nausea, vomiting, arthralgia, myalgia, dizziness, hyperkinesia, abdominal or back pain, hypotension, hypertension, increased LDH, somnolence, vasodilation, pruritus, rash and sweating. All adverse reactions listed occurred in ≤ 2% of WinRho® SDF doses administered in clinical trials.
Adverse reactions observed in the use of WinRho® SDF for Suppression of Rh Isoimmunization are < 0.1% in Rho(D)-negative individuals.
6.1 Clinical Trials Experiences
Because clinical studies are conducted under different protocols and widely varying conditions, adverse reaction rates observed in the clinical trials of a specific drug product cannot be directly compared to rates in clinical trials of another drug, and may not reflect rates observed in practice.
Treatment of ITP
The safety of WinRho® SDF was evaluated in clinical trials (n=161) in children and adults with acute and chronic ITP and adults and children with ITP secondary to HIV. Overall, 417 adverse events were reported by 91 patients (57%). The most common adverse events were headache (14% of the patients), fever (11% of the patients) and asthenia (11% of the patients). A total of 117 adverse drug reactions were reported by 46 patients (29%). Headache, chills, and fever were the most common related adverse events (Table 5). With respect to safety profile per administration, 60/848 (7%) of WinRho® SDF infusions had at least one adverse reaction. The most common adverse reactions were headache (19 infusions; 2%), chills (14 infusions; < 2%), and fever (9 infusions; 1%).
Table 5: Adverse Drug Reactions with an Incidence ≥ 5% of Patients Body System Adverse Event All Studies Children Adults # of Patients (%) Body as a Whole Headache 18 (11) 8 (11) 10 (12) Chills 13 (8) 4 (5) 9 (10) Fever 9 (6) 5 (7) 4 (5) Asthenia 6 (4) 2 (3) 4 (5) Infection 4 (3) 4 (5) 0 (0) Nervous System Dizziness 6 (4) 2 (3) 4 (5) In four clinical trials of patients treated with the recommended initial intravenous dose of 250 IU/kg (50 mcg/kg), the mean maximum decrease in hemoglobin was 1.70 g/dL (range: +0.40 to -6.1 g/dL). At a reduced dose, ranging from 125 to 200 IU/kg (25 to 40 mcg/kg), the mean maximum decrease in hemoglobin was 0.81 g/dL (range: +0.65 to -1.9 g/dL). Only 5/137 (3.7%) of patients had a maximum decrease in hemoglobin of greater than 4 g/dL (range: -4.2 to -6.1 g/dL).
Suppression of Rh Isoimmunization
In the clinical trial of 1,186 Rho(D)-negative pregnant women, no adverse reactions were reported to Rho(D) IGIV.
6.2 Post-marketing Experience
The following adverse reactions listed by body system have been identified during the post-approval use of WinRho® SDF. Because post-marketing adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to product exposure.
Intravascular hemolysis (IVH) leading to death has been reported in patients treated with WinRho® SDF for immune thrombocytopenic purpura (ITP).
Serious complications including severe anemia, acute renal insufficiency, renal failure and disseminated intravascular coagulation (DIC) have also been reported.
Blood and Lymphatic: Intravascular hemolysis, disseminated Intravascular coagulation, hemoglobinemia Cardiac: Cardiac arrest, cardiac failure, myocardial infarction, tachycardia Gastrointestinal: Nausea General: Chest pain, fatigue, edema, pain Hepatobilliary: Jaundice Immune System: Anaphylactic reaction/shock, hypersensitivity, injection site reaction including induration, pruritus and/or swelling Musculoskeletal: Myalgia, muscle spasm, pain in extremities Renal: Renal failure, anuria, chromaturia, hematuria, hemoglobinuria Respiratory: Acute respiratory distress syndrome, dyspnea, transfusion related acute lung injury Skin: Hyperhidrosis, pruritus, rash Healthcare professionals should report serious adverse reactions following the administration of WinRho® SDF to Saol Therapeutics Inc. at 1-833-644-4216 or FDA’s MedWatch reporting system by phone (1-800-FDA-1088).
-
7 DRUG INTERACTIONS
7.1 Live Virus Vaccines
Administration of WinRho® SDF concomitantly with other drugs has not been evaluated. Passive transfer of antibodies may transiently impair the immune response to live attenuated virus vaccines such as measles, mumps, rubella, and varicella (see Patient Counseling Information [17.1]). Do not give immunization with live vaccines within 3 months after WinRho® SDF administration.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
For the treatment of ITP, there is no human data or animal data available to establish the presence or absence of drug-associated risk.
When administered to pregnant women in a clinical trial to evaluate WinRho® SDF for suppression of Rh isoimmunization [see Clinical Studies (14.2)] following dosing regimens similar to Table 2 [see Dosing and Administration (2.1)], WinRho® SDF was not shown to harm the fetus or newborn.12
8.2 Lactation
Risk Summary
There is no information regarding the presence of WinRho® SDF in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for WinRho® SDF and any potential adverse effects on the breastfed infant from WinRho® SDF or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of WinRho® SDF has been evaluated for the treatment of chronic or acute ITP in children and in children (<16 years of age) with ITP secondary to HIV infection [see Adverse Reactions (6.2)]. The dosing recommendation in the treatment of children with ITP is the same as in adults [see Dosage and Administration (2.1)].
8.5 Geriatric Use
Clinical studies of WinRho® SDF did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Post marketing clinical experience suggests that patients of advanced age (age over 65) with co-morbid conditions including but not limited to cardio-respiratory decompensation, renal failure or insufficiency or prothrombotic conditions are at increased risk of developing serious complications from acute hemolytic reactions such as IVH. Patients receiving doses in excess of 300 IU/kg of WinRho® SDF may also be at an increased risk of developing increased hemolysis. Fatal outcomes associated with IVH and its complications have occurred most frequently in patients of advanced age (age over 65) with co-morbid conditions.
Given the prevalence of co-morbid conditions and concomitant drug therapy in geriatric patients, consider starting at the low end of the dosing range when using WinRho® SDF in this population.
-
10 OVERDOSAGE
Treatment of ITP and Suppression of Rh Isoimmunization
In post-marketing spontaneous reporting, there has been a limited number of medication error reports related to dosage calculations in which higher doses than that recommended for WinRho® SDF were administered (doses > 60 mcg/kg). Signs and laboratory findings of overdosage in Rh positive (ITP) patients have included hemoglobin decreases in excess of 1.2 g/dL. For the suppression of Rh isoimmunization, hemolytic reactions have been reported in cases of mis-matched blood transfusions where very large doses of WinRho® SDF were administered.
In one ITP case report that involved an overdose due to confusion between mcg and international unit (IU), a patient with significant co-morbidities developed IVH and had a fatal outcome. In the event of overdose, monitor patients closely for signs and symptoms of hemolysis and initiate symptomatic and supportive treatment.
-
11 DESCRIPTION
WinRho® SDF is a sterile, liquid gamma globulin (IgG) fraction containing antibodies to the Rho(D) antigen (D antigen). WinRho® SDF is to be administered intravenously for the treatment of ITP and either intravenously or intramuscularly for the suppression of Rh isoimmunization.
WinRho® SDF is prepared from human plasma by an anion-exchange column chromatography method. The manufacturing process includes two steps implemented specifically for viral clearance. The solvent detergent treatment step (using tri-n-butyl phosphate and octoxynol) is effective in inactivating lipid enveloped viruses such as hepatitis B, hepatitis C, and HIV. Virus filtration, using a 20N virus filter, is effective in the removal of some non-lipid enveloped viruses. These two processes are designed to increase product safety by reducing the risk of transmission of enveloped and non-enveloped viruses, respectively. In addition to the two specific steps, the anion-exchange chromatography step contributes to the removal of small non-lipid enveloped viruses.
The inactivation and reduction of known enveloped and non-enveloped model viruses were validated in laboratory studies as summarized in Table 6.
Table 6: Virus Reduction Values Obtained Through Validation Studies * The PRV was retained by the 0.1 µm pre-filter during the virus validation. Since manufacturing employs a 0.1 µm pre-filter before the 20N filter, the claim of ≥5.6 reduction is considered applicable.
Abbreviations:
HIV-1: human immunodeficiency virus-1; relevant virus for human immunodeficiency virus-1 and model for HIV-2.
BVDV: bovine viral diarrhea virus; model virus for hepatitis C virus (HCV) and West Nile virus (WNV)
PRV: pseudorabies virus; model for large enveloped DNA viruses, including herpes
HAV: human hepatitis A virus; relevant virus for HAV and model for small non-enveloped viruses in general
EMC: encephalomyocarditis virus; model for HAV and for small non-enveloped viruses in general
MMV: murine minute virus; model for human parvovirus B19 and for small non-enveloped viruses in general
PPV: porcine parvovirus; model for human parvovirus B19 and for small non-enveloped viruses in general
n.e.: not evaluated
Enveloped Non-Enveloped Genome RNA DNA RNA DNA Virus HIV-1 BVDV PRV HAV EMC MMV PPV Family retro flavi herpes picorna parvo Size (nm) 80-100 50-70 120-200 25-30 30 20-25 18-24 Anion Exchange Chromatography (partitioning) n.e. 2.3 n.e. 3.4 n.e. 20N Filtration (size exclusion) ≥ 4.7 ≥ 3.5 ≥ 5.6* n.e. 4.8 n.e. 4.1 Solvent/Detergent (inactivation) ≥ 4.7 ≥ 7.3 ≥ 5.5 n.e. Total Reduction (log10) ≥ 9.4 ≥ 10.8 ≥ 11.1 2.3 4.8 3.4 4.1 The product potency is expressed in international units (IU) by comparison to the World Health Organization (WHO) standard. In the past, a full dose of Rho(D) Immune Globulin (Human) has traditionally been referred to as a “300 microgram (mcg)” dose. Potency and dosing recommendations are now expressed in IU by comparison to the WHO anti-Rho(D) standard. The conversion of mcg to IU is: 1 mcg = 5 IU. A 1,500 IU (300 mcg) vial contains sufficient anti-Rho(D) to effectively suppress the immunizing potential of approximately 17 mL of Rho(D) (D-positive) RBCs.
The liquid formulation is stabilized with 10% maltose and 0.03% polysorbate 80. There are no preservatives in the formulation. WinRho® SDF does not contain mercury. This product contains ≤ 40 mcg/mL IgA.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Treatment of ITP
WinRho® SDF has been shown to increase platelet counts in non-splenectomized, Rho(D)-positive patients with ITP. Platelet counts usually rise within one to two days and peak within seven to 14 days after initiation of therapy. The mechanism of action is not completely understood, but is thought to be due to the formation of anti-Rho(D)-coated RBC complexes, which are preferentially removed by the reticuloendothelial system, particularly the spleen. This results in Fc receptor blockade, thus sparing antibody-coated platelets.9,10
Suppression of Rh Isoimmunization
The mechanism by which Rho(D) immune globulin suppresses immunization to Rho(D)-positive RBCs is not completely understood.
WinRho® SDF when administered within 72 hours of a full-term delivery of a Rho(D)-positive infant by a Rho(D) negative mother will reduce the incidence of Rh isoimmunization from 12-13% to 1-2%. The 1-2% is, for the most part, due to isoimmunization during the last trimester of pregnancy. When treatment is given both antenatally, at 28-weeks gestation, and postpartum, the Rh immunization rate drops to about 0.1%.13,14
When 600 IU (120 mcg) of WinRho® SDF is administered to pregnant women, passive anti-Rho(D) antibodies are not detectable in the circulation for more than six weeks and therefore a dose of 1,500 IU (300 mcg) should be used for antenatal administration.
12.2 Pharmacodynamics
In a clinical study with Rho(D)-negative volunteers (nine males and one female), Rho(D)-positive RBCs were completely cleared from the circulation within 8 hours of intravenous administration of WinRho® SDF. There was no indication of Rh isoimmunization of these subjects at six months after the clearance of the Rho(D)-positive RBCs.
12.3 Pharmacokinetics
IM versus IV Administration (Lyophilized Powder)
In a clinical study involving Rho(D)-negative volunteers, two subjects received 600 IU (120 mcg) WinRho® SDF by intravenous (IV) administration and two subjects received this dose by intramuscular (IM) administration. Peak levels (36 to 48 ng/mL) were reached within two hours of IV administration and peak levels (18 to 19 ng/mL) were reached at five to 10 days after IM administration. Although no statistical comparisons were made, the calculated areas under the curve were comparable for both routes of administration. The t½ for anti-Rho(D) was about 24 days following IV administration and about 30 days following IM administration.
Lyophilized Powder versus Liquid Formulation
In two comparative pharmacokinetics studies, 101 volunteers were administered the liquid or lyophilized formulation of WinRho® SDF intravenously (n=41) or intramuscularly (n=60). The formulations were bioequivalent following IV administration based on area under the curve to 84 days and had comparable pharmacokinetics following IM administration. The average peak concentrations (Cmax) of anti-Rho(D) for both formulations were comparable following IV or IM administration and occurred within 30 minutes or 2-4 days of administration, respectively. Both formulations also had similar elimination half-lives (t½) following IV or IM administration.
-
14 CLINICAL STUDIES
14.1 Treatment of ITP
Efficacy was documented in four subgroups of patients with ITP:
Childhood Chronic ITP
In an open-label, single arm, multicenter study, 24 non-splenectomized, Rho(D)-positive children with ITP of greater than six-months duration were treated initially with 250 IU/kg (50 mcg/kg) WinRho® SDF [125 IU/kg (25 mcg/kg) on days 1 and 2, with subsequent doses ranging from 125 to 275 IU/kg (25 to 55 mcg/kg)]. Response was defined as a platelet increase to at least 50,000/mm3 and a doubling of the baseline. Nineteen of 24 patients responded for an overall response rate of 79%, an overall mean peak platelet count of 229,400/mm3 (range 43,300 to 456,000), and a mean duration of response of 36.5 days (range 6 to 84).15
Childhood Acute ITP
A multicenter, randomized, controlled trial comparing WinRho® SDF to high dose and low dose Immune Globulin Intravenous (Human) (IGIV) and prednisone was conducted in 146 non-splenectomized, Rho(D)-positive children with acute ITP and platelet counts less than 20,000/mm3. Of 38 patients receiving WinRho® SDF [125 IU/kg (25 mcg/kg) on days 1 and 2], 32 patients (84%) responded (platelet count ≥ 50,000/mm3) with a mean peak platelet count of 319,500/mm3 (range 61,000 to 892,000), with no statistically significant differences compared to other treatment arms. The mean times to achieving ≥ 20,000/mm3 or ≥ 50,000/mm3 platelets for patients receiving WinRho® SDF were 1.9 and 2.8 days respectively. When comparing the different therapies for time to platelet count ≥ 20,000/mm3 or ≥ 50,000/mm3, no statistically significant differences among treatment groups were detected, with a range of 1.3 to 1.9 days and 2.0 to 3.2 days, for IGIV and prednisone respectively.16,17
Adult Chronic ITP
Twenty-four non-splenectomized Rho(D)-positive adults with ITP of greater than six-months duration and platelet counts < 30,000/mm3 or requiring therapy were enrolled in a single-arm, open-label trial were treated with 100 to 375 IU/kg (20 to 75 mcg/kg) WinRho® SDF [mean dose 231 IU/kg (46.2 mcg/kg)]. Twenty-one of 24 patients responded (increase ≥ 20,000/mm3) during the first two courses of therapy for an overall response rate of 88% with a mean peak platelet count of 92,300/mm3 (range 8,000 to 229,000).18,19
ITP Secondary to HIV Infection
Eleven children and 52 adults, who were non-splenectomized and Rho(D)-positive, with all Walter Reed classes of HIV infection and ITP, with initial platelet counts of ≤ 30,000/mm3 or requiring therapy, were treated with 100 to 375 IU/kg (20 to 75 mcg/kg) WinRho® SDF in an open label trial. WinRho® SDF was administered for an average of 7.3 courses (range 1 to 57) over a mean period of 407 days (range 6 to 1,952). Fifty-seven of 63 patients responded (increase ≥ 20,000/mm3) during the first six courses of therapy for an overall response rate of 90%. The overall mean change in platelet count for six courses was 60,900/mm3 (range -2,000 to 565,000), and the mean peak platelet count was 81,700/mm3 (range 16,000 to 593,000).18-20
14.2 Suppression of Rh Isoimmunization
A study was conducted in 1,186 non-sensitized, Rho(D)-negative pregnant women in cases in which the blood types of the fathers were Rho(D)-positive or unknown. WinRho® SDF was administered according to one of three regimens: 1) 93 women received 600 IU (120 mcg) at 28 weeks; 2) 131 women received 1,200 IU (240 mcg) each at 28 and 34 weeks; 3) 962 women received 1,200 IU (240 mcg) at 28 weeks. All women received a postnatal administration of 600 IU (120 mcg) if the newborn was found to be Rho(D)-positive. Of 1,186 women who received antenatal WinRho® SDF, 806 were given WinRho® SDF postnatal following the delivery of a Rho(D)-positive infant, of which 325 women underwent testing at six months after delivery for evidence of Rh isoimmunization. Of these 325 women, 23 would have been expected to display signs of Rh isoimmunization, however, none was observed (p <0.001 in a Chi-square test of significance of difference between observed and expected isoimmunization in the absence of WinRho® SDF).
-
15 REFERENCES
- Provan D, et al.: International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010; 115:168-186.
- Gupta N, Ahmed I, Nissel-Horowitz S, Patel D, Mehrotra B. Intravenous gammaglobulin-associated acute renal failure. Am J Hematol 2001; 66:151-152
- Dalakas MC. High-dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic events. Neurology 1994; 44:223-226.
- Woodruff RK, et al.: Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet 1986; 2:217-218.
- Rushin J, Rumsey, DH, Ewing, CA, Sandler, SG. Detection of multiple passively acquired alloantibodies following infusions of IV Rh immune globulins. Transfusion Vol. 40, May 2000.
- Rizk A, et al.: Transfusion-related acute lung injury after the infusion of IVIG. Transfusion 2001; 41:264-8.
- Gaines AR. Acute onset hemoglobinemia and/or hemoglobinuria and sequalae following Rho(D) immune globulin intravenous administration in immune thrombocytopenic purpura patients. Blood 2000; 95(8): 2523-2529.
- Gaines AR. Disseminated intravascular coagulation associated with acute hemoglobinemia and/or hemoglobinuria following Rho(D) immune globulin intravenous administration for immune thrombocytopenic purpura. Blood 2005; 106(5); 1532-7.
- Ballow, M: Mechanisms of action of intravenous immunoglobulin therapy and potential use in autoimmune connective tissue diseases. Cancer. 1991; 68:1430-1436.
- Kniker, WT: Immunosuppressive agents, γ-globulin, immunomodulation, immunization, and apheresis. J. Aller. Clin. Immunol. 1989; 84:1104-1106.
- Lazarus AH, Crow AR. Mechanism of action of IVIG and anti-D in ITP. Transfus Apheresis Sci 2003; 28:249-255.
- Bowman JM. The prevention of Rh immunization. Transfus Med Rev 1988; 2(3):129-150
- Bowman, JM, and Pollock, JM: Failures of intravenous Rh immune globulin prophylaxis: An analysis of the reasons for such failures. Trans. Med. Rev. 1987; 1:101-111.
- Bowman, JM: Antenatal suppression of Rh alloimmunization. Clin Obstet. & Gynec. 1991; 34:296-303.
- Andrew, M, et al.: A multicenter study of the treatment of childhood chronic idiopathic thrombocytopenic purpura with anti-D. J Pediatrics 1992; 120:522-527.
- Blanchette, V, et al.: Randomised trial of intravenous immunoglobulin G, intravenous anti-D, and oral prednisone in childhood acute immune thrombocytopenic purpura. Lancet 1994; 344: 703-707.
- Zunich KM, et al. Intravenous anti-D immunoglobulin for childhood acute immune thrombocytopenic purpura. Lancet 1995; 346:1363-5.
- Scaradavou A, et al.: Intravenous anti-D treatment of immune thrombocytopenic purpura: experience in 272 patients. Blood 1997; 89:2689-700.
- Bussel, JB, et al.: Intravenous anti-D treatment of immune thrombocytopenic purpura: Analysis of efficacy, toxicity, and mechanism of effect. Blood 1991; 77: 1884-1893.
- Zunich KM, et al.: Treatment of human immunodeficiency virus-related thrombocytopenia with intravenous anti-rhesus D immunoglobulin. Clin Infect Dis 1996; 22:1129-30.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
- Store at 2 to 8°C (36 to 46°F)
- Do not freeze
- Do not use after expiration date
- Protect from light
WinRho® SDF is available in packages containing:
NDC Number Product Description 70257-312-51 A carton containing a single dose vial of 600 IU (120 mcg)
anti-Rho(D) IGIV and a package insert70257-330-51 A carton containing a single dose vial of 1,500 IU (300 mcg)
anti-Rho(D) IGIV and a package insert70257-350-51 A carton containing a single dose vial of 2,500 IU (500 mcg)
anti-Rho(D) IGIV and a package insert70257-310-51 A carton containing a single dose vial of 5,000 IU (1,000 mcg)
anti-Rho(D) IGIV and a package insert70257-300-51 A carton containing a single dose vial of 15,000 IU (3,000 mcg)
anti-Rho(D) IGIV and a package insert -
17 PATIENT COUNSELING INFORMATION
Information for Patients
See FDA-Approved Patient Labeling
-
ITP and Suppression of Rh Isoimmunization
- Inform patients of the early signs of hypersensitivity reactions to WinRho® SDF including hives, generalized urticaria, chest tightness, wheezing, hypotension, and anaphylaxis.
- Advise patients to notify their physicians if they experience any of the above symptoms.
-
Blood Glucose Monitoring
- Advise patients that the maltose contained in WinRho® SDF can interfere with some types of blood glucose monitoring systems.
- Advise patients to use only testing systems that are glucose specific for monitoring blood glucose levels as the interference of maltose could result in falsely elevated glucose readings. This could lead to untreated hypoglycemia or to inappropriate insulin administration, resulting in life-threatening hypoglycemia.
-
Transmittable Infectious Agents
- Inform patients that WinRho® SDF is prepared from human plasma and may contain infectious agents (e.g., viruses and, theoretically, the CJD agent) that can cause disease. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of current virus infections, and by inactivating and/or removing certain viruses during manufacturing.
- Advise patients to report any symptoms that concern them and that may be related to viral infections.
-
Live Virus Vaccines
- Advise patients that WinRho® SDF may impair the effectiveness of certain live virus vaccines (e.g., measles, rubella, mumps, and varicella).
- Instruct patients to notify their treating physician of this potential interaction when they are receiving vaccinations.
-
Immune Thrombocytopenic Purpura (ITP)
- Instruct patients being treated with WinRho® SDF for ITP to immediately report symptoms of intravascular hemolysis including back pain, shaking chills, fever, discolored urine, decreased urine output, sudden weight gain, fluid retention/edema, and/or shortness of breath to their physicians.
- Prior to discharge, instruct patients to continue to self-monitor for the signs and symptoms of IVH over 72 hours, especially for discoloration of urine, and to seek medical attention immediately in the event that signs/symptoms of IVH occur following WinRho® SDF administration.
- Laboratory Tests
Assess renal function in patients judged to be at an increased risk of developing acute renal failure, including measurement of BUN and serum creatinine, before the initial infusion of WinRho® SDF.
17.1 FDA Approved Patient Labeling – (Patient Prescribing Information)
WinRho® SDF
[pronounced win – row –S-D-F]
You should read this leaflet carefully each time before you are scheduled to receive a treatment for your Immune Thrombocytopenic Purpura (ITP) with WinRho® SDF. This letter is a summary of the important information you need to know about your medicine, and does not take the place of talking with your doctor and does not contain all of the information available about WinRho® SDF. If you have any questions after reading this leaflet, make sure you ask your doctor or nurse.
1. What is the most important information I should know about WinRho® SDF?
Some patients taking WinRho® SDF for immune thrombocytopenic purpura (ITP) have had severe, life threatening bleeding and clotting problems. For this reason, you need to remain under observation for at least 8 hours following each treatment with WinRho® SDF and your doctor will ask you to take blood and urine tests before and after infusion with WinRho® SDF.
Some patients taking WinRho® SDF have had problems with their kidneys and other organs.
Problems usually occur within 4 to 8 hours after getting an infusion. Tell your doctor or healthcare provider right away if you have any of the following signs or symptoms after getting a WinRho® SDF infusion:
- back pain
- shaking
- chills
- fever
- dark or oddly colored urine
- decreased urination
- swelling or sudden weight gain
- shortness of breath
- rash
- dizziness
Continue monitoring for these signs and symptoms for 72 hours after each treatment with WinRho® SDF.
WinRho® SDF contains maltose, which can give false readings on some glucose testing meters. If you are diabetic, ask your doctor what types of glucose testing meters can be used safely while you are getting WinRho® SDF.
2. What is WinRho® SDF?
WinRho® SDF is a protein product, called an “immune globulin,” which is made from human plasma. It has antibodies to the “D” antigen that people with “Rh-positive” blood have in their blood.
WinRho® SDF is used to increase the number of platelets in the blood of Rh-positive people who have a problem called immune thrombocytopenic purpura (ITP). People with ITP bruise and bleed easily because they have a very low number of platelets in their blood.
WinRho® SDF is also used to treat Rh-negative girls and women who need a blood transfusion using Rh-positive blood and/or are carrying a Rh-positive baby.
3. Who should not use WinRho® SDF?
You should not use WinRho® SDF for any treatment if you:
- have ever had a severe allergic reaction (such as trouble breathing, hives, passing out) after getting any blood product or blood product transfusion.
- have an immune globulin A (IgA) deficiency.
You should not use WinRho® SDF for treatment of ITP if you:
- have Rh-negative blood.
- have had your spleen removed.
- have a problem called “autoimmune hemolytic anemia.”
- have other pre-existing bleeding problems.
4. How will I get WinRho® SDF?
Your doctor will give you WinRho® SDF as an injection into your vein. For the treatment of ITP, it will usually take 3 to 5 minutes for an injection. Your doctor will decide if you need one or more injections.
For protection against Rh-positive blood, your doctor may decide to give you WinRho® SDF as a shot in your arm or thigh.
5. What should I avoid while using WinRho® SDF?
WinRho® SDF may interfere with your immune response to routine immunizations. Tell your doctor if you have recently been vaccinated or are planning to be vaccinated.
WinRho® SDF can interfere with certain blood tests. It is important to tell the person taking your blood and the doctor that you got WinRho® SDF.
6. What are the possible or reasonably likely side effects of WinRho® SDF?
The most common side effects of WinRho® SDF are
- headache
- chills
- fever
- weakness
- diarrhea
- nausea and vomiting
- achy muscles
- feeling light-headed or dizziness
- fainting
- flushing
- rash
- sweating
Tell your doctor right away if you have:
- a fever over 100°F
- shaking or chills that continue or get worse
- a painful lump or swelling (because this may be a sign of a blood clot)
- bruising that is increasing in diameter (because this may be a sign of a clotting problem)
- oddly colored urine
- trouble urinating
- severe back pain
- severe abdominal pain
- swelling, especially around the ankles
- hives
- shortness of breath
Talk to your doctor about any side effects that concern you.
Additional prescribing information is available to healthcare professionals.
7. What other information do I need to know about WinRho® SDF?
WinRho® SDF is made from human plasma. Donors are carefully screened and the plasma is carefully cleaned, but it does have a very small risk of giving you viruses from the donor. Talk to your doctor if you have any symptoms that concern you.
You may report side effects to Saol Therapeutics Inc. at 1-833-644-4216 or FDA’s MedWatch reporting system by phone (1-800-FDA-1088)
WinRho® SDF [Rho(D) Immune Globulin Intravenous (Human)] Sterile Solution for Injection and any and all Saol brand, product, service and feature names, logos, slogans are trademarks or registered trademarks of Saol. All rights reserved.
Distributed by:
Saol Therapeutics Inc.
Roswell, GA 30076US License No. 2098
-
ITP and Suppression of Rh Isoimmunization
- PRINCIPAL DISPLAY PANEL - NDC: 70257-330-11 - 1500 IU Vial Label
- PRINCIPAL DISPLAY PANEL - NDC: 70257-330-51 - 1500 IU Carton Label
- PRINCIPAL DISPLAY PANEL - NDC: 70257-350-02 - 2500 IU Vial Label
- PRINCIPAL DISPLAY PANEL - NDC: 70257-350-51 - 2500 IU Carton Label
- PRINCIPAL DISPLAY PANEL - NDC: 70257-310-04 - 5000 IU Vial Label
- PRINCIPAL DISPLAY PANEL - NDC: 70257-310-51 - 5000 IU Carton Label
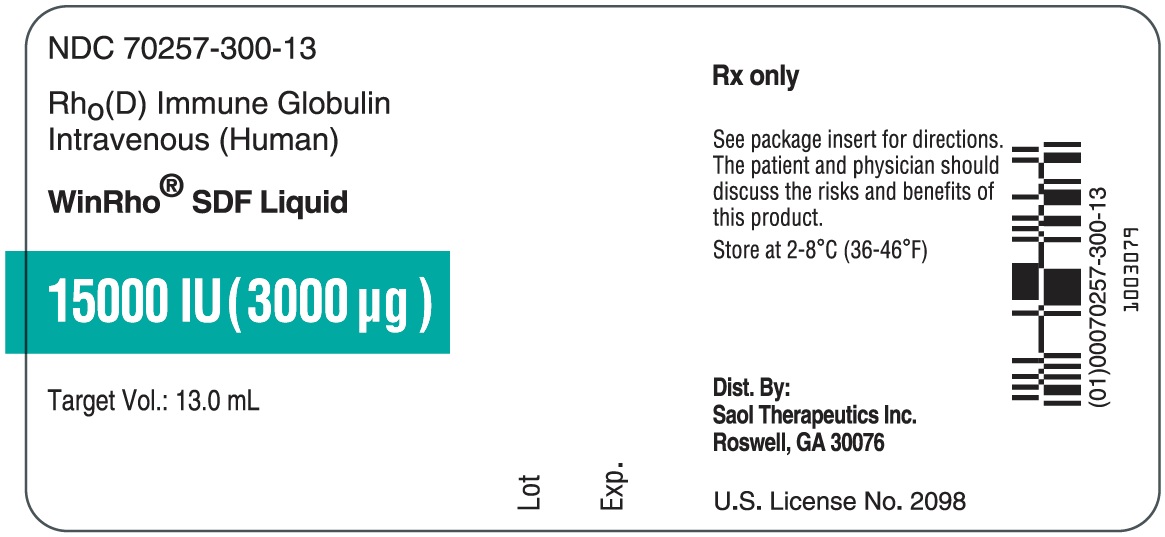
- PRINCIPAL DISPLAY PANEL - NDC: 70257-300-13 - 15000 IU Vial Label
- PRINCIPAL DISPLAY PANEL - NDC: 70257-300-51 - 15000 IU Carton Label
-
INGREDIENTS AND APPEARANCE
WINRHO SDF
rho(d) immune globulin intravenous (human) injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70257-330 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 1500 [iU] Inactive Ingredients Ingredient Name Strength MALTOSE (UNII: XJ6S9RV06F) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70257-330-51 1 in 1 CARTON 03/01/2019 1 NDC: 70257-330-11 1 in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103649 03/01/2019 WINRHO SDF
rho(d) immune globulin intravenous (human) injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70257-350 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 2500 [iU] Inactive Ingredients Ingredient Name Strength MALTOSE (UNII: XJ6S9RV06F) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70257-350-51 1 in 1 CARTON 03/01/2019 1 NDC: 70257-350-02 1 in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103649 03/01/2019 WINRHO SDF
rho(d) immune globulin intravenous (human) injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70257-310 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 5000 [iU] Inactive Ingredients Ingredient Name Strength MALTOSE (UNII: XJ6S9RV06F) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70257-310-51 1 in 1 CARTON 03/01/2019 1 NDC: 70257-310-04 1 in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103649 03/01/2019 WINRHO SDF
rho(d) immune globulin intravenous (human) injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70257-300 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 15000 [iU] Inactive Ingredients Ingredient Name Strength MALTOSE (UNII: XJ6S9RV06F) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70257-300-51 1 in 1 CARTON 03/01/2019 1 NDC: 70257-300-13 1 in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103649 03/01/2019 Labeler - Saol Therapeutics Inc. (080040201)
Trademark Results [WinRho SDF]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 WINRHO SDF 75327094 2183956 Dead/Cancelled |
CANGENE CORPORATION 1997-07-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.