MEFLOQUINE HYDROCHLORIDE tablet
Mefloquine Hydrochloride by
Drug Labeling and Warnings
Mefloquine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Chartwell RX, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
WARNING
Mefloquine may cause neuropsychiatric adverse reactions that can persist after mefloquine has been discontinued. Mefloquine should not be prescribed for prophylaxis in patients with major psychiatric disorders. During prophylactic use, if psychiatric or neurologic symptoms occur, the drug should be discontinued and an alternative medication should be substituted (see WARNINGS).
-
DESCRIPTION
Mefloquine hydrochloride is an antimalarial agent available as 250 mg tablets of mefloquine hydrochloride (equivalent to 228.0 mg of the free base) for oral administration.
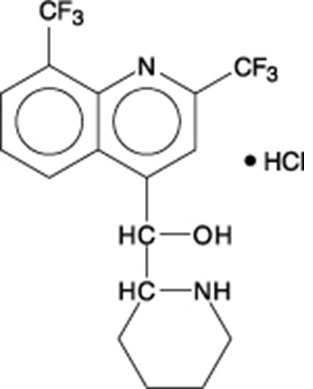
Mefloquine hydrochloride is a 4-quinolinemethanol derivative with the specific chemical name of (R*, S*)-(±)-α-2-piperidinyl-2,8-bis (trifluoromethyl)-4-quinolinemethanol hydrochloride. It is a 2-aryl substituted chemical structural analog of quinine. The drug is a white to almost white crystalline compound, slightly soluble in water.
Mefloquine hydrochloride has a calculated molecular weight of 414.78 and the following structural formula:
Mefloquine Hydrochloride Tablets USP, 250 mg meets USP Dissolution Test 2.
The inactive ingredients are crospovidone, lactose monohydrate, low-substituted hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose, pregelatinized starch and talc.
- CLINICAL PHARMACOLOGY
- Pharmacokinetics
-
Absorption
The absolute oral bioavailability of mefloquine has not been determined since an intravenous formulation is not available. The bioavailability of the tablet formation compared with an oral solution was over 85%. The presence of food significantly enhances the rate and extent of absorption, leading to about a 40% increase in bioavailability. In healthy volunteers, plasma concentrations peak 6 to 24 hours (median, about 17 hours) after a single dose of mefloquine. In a similar group of volunteers, maximum plasma concentrations in mcg/L are roughly equivalent to the dose in milligrams (for example, a single 1000 mg dose produces a maximum concentration of about 1000 mcg/L). In healthy volunteers, a dose of 250 mg once weekly produces maximum steady-state plasma concentrations of 1000 to 2000 mcg/L, which are reached after 7 to 10 weeks.
-
Distribution
In healthy adults, the apparent volume of distribution is approximately 20 L/kg, indicating extensive tissue distribution. Mefloquine may accumulate in parasitized erythrocytes. Experiments conducted in vitrowith human blood using concentrations between 50 and 1000 mg/mL showed a relatively constant erythrocyte-to-plasma concentration ratio of about 2 to 1. The equilibrium reached in less than 30 minutes was found to be reversible. Protein binding is about 98%.
Mefloquine crosses the placenta. Excretion into breast milk appears to be minimal (see PRECAUTIONS: Nursing Mothers).
-
Metabolism
Mefloquine is extensively metabolized in the liver by the cytochrome P450 system. In vitroand in vivostudies strongly suggested that CYP3A4 is the major isoform involved.
Two metabolites of mefloquine have been identified in humans. The main metabolite, 2,8- bis-trifluoromethyl-4-quinoline carboxylic acid, is inactive in Plasmodium falciparum. In a study in healthy volunteers, the carboxylic acid metabolite appeared in plasma 2 to 4 hours after a single oral dose. Maximum plasma concentrations of the metabolite, which were about 50% higher than those of mefloquine, were reached after 2 weeks. Thereafter, plasma levels of the main metabolite and mefloquine declined at a similar rate. The area under the plasma concentration-time curve (AUC) of the main metabolite was 3 to 5 times larger than that of the parent drug. The other metabolite, an alcohol, was present in minute quantities only.
-
Elimination
In several studies in healthy adults, the mean elimination half-life of mefloquine varied between 2 and 4 weeks, with an average of about 3 weeks. Total clearance, which is essentially hepatic, is in the order of 30 mL/min. There is evidence that mefloquine is excreted mainly in the bile and feces. In volunteers, urinary excretion of unchanged mefloquine and its main metabolite under steady-state condition accounted for about 9% and 4% of the dose, respectively. Concentrations of other metabolites could not be measured in the urine.
-
Pharmacokinetics in Special Clinical Situations
Children and the Elderly
No relevant age-related changes have been observed in the pharmacokinetics of mefloquine. Therefore, the dosage for children has been extrapolated from the recommended adult dose.
No pharmacokinetic studies have been performed in patients with renal insufficiency since only a small proportion of the drug is eliminated renally. Mefloquine and its main metabolite are not appreciably removed by hemodialysis. No special chemoprophylactic dosage adjustments are indicated for dialysis patients to achieve concentrations in plasma similar to those in healthy persons.
Although clearance of mefloquine may increase in late pregnancy, in general, pregnancy has no clinically relevant effect on the pharmacokinetics of mefloquine.
The pharmacokinetics of mefloquine may be altered in acute malaria.
Pharmacokinetic differences have been observed between various ethnic populations. In practice, however, these are of minor importance compared with host immune status and sensitivity of the parasite.
During long-term prophylaxis (>2 years), the trough concentrations and the elimination half-life of mefloquine were similar to those obtained in the same population after 6 months of drug use, which is when they reached steady state.
In vitroand in vivostudies showed no hemolysis associated with glucose-6-phosphate dehydrogenase deficiency (see ANIMAL TOXICOLOGY).
-
Microbiology
Mechanism of Action
Mefloquine is an antimalarial agent which acts as a blood schizonticide. Its exact mechanism of action is not known.
Activity In Vitro and In Vivo
Mefloquine is active against the erythrocytic stages of Plasmodiumspecies (see INDICATIONS AND USAGE). However, the drug has no effect against the exoerythrocytic (hepatic) stages of the parasite. Mefloquine is effective against malaria parasites resistant to chloroquine (see INDICATIONS AND USAGE).
Drug Resistance
Strains of P. falciparumwith decreased susceptibility to mefloquine can be selected in vitroor in vivo. Resistance of P. falciparumto mefloquine has been reported in areas of multi-drug resistance in South East Asia. Increased incidences of resistance have also been reported in other parts of the world.
- Cross Resistance
- INDICATIONS AND USAGE
-
Treatment of Acute Malaria Infections
Mefloquine hydrochloride tablets are indicated for the treatment of mild to moderate acute malaria caused by mefloquine-susceptible strains of P. falciparum(both chloroquine-susceptible and resistant strains) or by Plasmodium vivax. There are insufficient clinical data to document the effect of mefloquine in malaria caused by P. ovaleor P. malariae.
Note:Patients with acute P. vivaxmalaria, treated with mefloquine, are at high risk of relapse because mefloquine does not eliminate exoerythrocytic (hepatic phase) parasites. To avoid relapse, after initial treatment of the acute infection with mefloquine, patients should subsequently be treated with an 8-aminoquinoline derivative (e.g., primaquine).
- Prevention of Malaria
-
CONTRAINDICATIONS
Use of mefloquine hydrochloride tablets is contraindicated in patients with a known hypersensitivity to mefloquine or related compounds (eg, quinine and quinidine) or to any of the excipients contained in the formulation. Mefloquine hydrochloride tablets should not be prescribed for prophylaxis in patients with active depression, a recent history of depression, generalized anxiety disorder, psychosis, schizophrenia or other major psychiatric disorders, or with a history of convulsions.
- WARNINGS
-
QTc Interval Prolongation and Drug Interactions
Halofantrine should not be administered with mefloquine or within 15 weeks of the last dose of mefloquine due to the risk of a potentially fatal prolongation of the QTc interval (see CLINICAL PHARMACOLOGY: Pharmacokinetics:Elimination).
Ketoconazole should not be administered with mefloquine or within 15 weeks of the last dose of mefloquine due to the risk of a potentially fatal prolongation of the QTc interval. Ketoconazole increases plasma concentrations and elimination half-life of mefloquine following co-administration (see CLINICAL PHARMACOLOGY: Pharmacokinetics:Eliminationand PRECAUTIONS: Drug Interactions).
Concomitant administration of mefloquine and quinine or quinidine may produce electrocardiographic abnormalities.
- Psychiatric and Neurologic Adverse Reactions
-
Psychiatric Adverse Reactions
Psychiatric symptoms ranging from anxiety, paranoia, and depression to hallucinations and psychotic behavior can occur with mefloquine use. Symptoms may occur early in the course of mefloquine use. In some cases, these symptoms have been reported to continue for months or years after mefloquine has been stopped. Cases of suicidal ideation and suicide have been reported.
Mefloquine should not be prescribed for prophylaxis in patients with active depression, generalized anxiety disorder, psychosis, or schizophrenia or other major psychiatric disorders. Mefloquine should be used with caution in patients with a previous history of depression.
During prophylactic use, the occurrence of psychiatric symptoms such as acute anxiety, depression, restlessness or confusion suggest a risk for more serious psychiatric disturbances or neurologic adverse reactions. In these cases, the drug should be discontinued and an alternative medication should be substituted.
-
Neurologic Adverse Reactions
Neurologic symptoms such as dizziness or vertigo, tinnitus, and loss of balance have been reported. These adverse reactions may occur early in the course of mefloquine use and in some cases have been reported to continue for months or years after mefloquine has been stopped. Dizziness or vertigo, tinnitus, and loss of balance have been reported to be permanent in some cases. During prophylactic use, if neurologic symptoms occur, the drug should be discontinued and an alternative medication should be substituted.
Caution should be exercised with regard to activities requiring alertness and fine motor coordination, such as driving, piloting aircraft, operating machinery, and deep-sea diving, while symptoms persist.
Mefloquine may increase the risk of convulsions in patients with epilepsy. The drug should therefore be prescribed only for curative treatment in such patients and only if there are compelling medical reasons for its use (see PRECAUTIONS: Drug Interactions).
Concomitant administration of mefloquine and quinine or chloroquine may increase the risk of convulsions.
-
Ocular Effects
Eye disorders, including but not limited to optic neuropathy and retinal disorders, have been reported during treatment with mefloquine. Any patient presenting with visual symptoms should be referred to the treating physician and an ophthalmologist as certain conditions (such as retinal disorders or optic neuropathy) may require stopping treatment with mefloquine (see PRECAUTIONS, ANIMAL TOXICOLOGY).
- PRECAUTIONS
- Hypersensitivity Reactions
- Use in Patients with Hepatic Impairment
-
Long-Term Use
This drug has been administered for longer than one year. If the drug is to be administered for a prolonged period, periodic evaluations including liver function tests and evaluations for neuropsychiatric effects should be performed (see WARNINGSand ADVERSE REACTIONS:Postmarketing). Periodic ophthalmic examinations are recommended (see WARNINGS).
-
Cardiac Effects
Parenteral studies in animals show that mefloquine, a myocardial depressant, possesses 20% of the anti-fibrillatory action of quinidine and produces 50% of the increase in the PR interval reported with quinine. The effect of mefloquine on the compromised cardiovascular system has not been evaluated. However, transitory and clinically silent ECG alterations have been reported during the use of mefloquine: alterations included sinus bradycardia, sinus arrhythmia, first degree AV-block, prolongation of the QTc interval and abnormal T waves (see also cardiovascular effects under PRECAUTIONS: Drug Interactionsand ADVERSE REACTIONS). The benefits of mefloquine therapy should be weighed against the possibility of adverse effects in patients with cardiac disease.
-
Drug Resistance and Cross-Resistance
Geographical drug resistance patterns of P. falciparumoccur and the preferred choice of malaria prophylaxis might be different from one area to another. For example, resistance of P. falciparumto mefloquine has been reported, predominantly in areas of multi-drug resistance in South-East Asia. Cross-resistance between mefloquine and halofantrine and cross-resistance between mefloquine and quinine have been observed in some regions.
- Agranulocytosis and Aplastic Anemia
- Laboratory Tests
-
Information for Patients
Medication Guide: As required by law, a Mefloquine Medication Guide is supplied to patients when mefloquine is dispensed. An information wallet card is also supplied to patients when mefloquine is dispensed. Patients should be instructed to read the Medication Guide when mefloquine is received and to carry the information wallet card with them when they are taking mefloquine. The complete text of the Medication Guide and information wallet can be printed as directed at the end of this document.
Patients should be advised:
- that malaria can be a life-threatening infection
- that mefloquine hydrochloride tablets are being prescribed to help prevent or treat this serious infection;
- that some patients are unable to take this medication because of side effects, including dizziness or vertigo and loss of balance, and it may be necessary to change medications. In some patients it has been reported that these symptoms may continue for months or years after discontinuation of the drug and can be permanent in some cases;
- that insomnia may occur
- that when used as prophylaxis, the first dose of mefloquine hydrochloride tablets should be taken one week prior to arrival in an endemic area;
- that if the patients experience psychiatric adverse reactions such as acute anxiety, depression, restlessness or confusion, or suicidal ideation, the drug should be discontinued and an alternative medication should be substituted.
- that no chemoprophylactic regimen is 100% effective, and protective clothing, insect repellents, and bed nets are important components of malaria prophylaxis;
- to seek medical attention for any febrile illness that occurs after return from a malaria area and to inform their physician that they may have been exposed to malaria.
-
Drug Interactions
Drug-drug interactions with mefloquine have not been explored in detail. There is one report of cardiopulmonary arrest, with full recovery, in a patient who was taking a beta blocker (propranolol) (see PRECAUTIONS: Cardiac Effects). The effects of mefloquine on the compromised cardiovascular system have not been evaluated. The benefits of mefloquine therapy should be weighed against the possibility of adverse effects in patients with cardiac disease.
-
Halofantrine
Halofantrine should not be administered with mefloquine or within 15 weeks of the last dose of mefloquine due to the risk of a potentially fatal prolongation of the QTc interval (see WARNINGS).
-
Other Antimalarial Drugs
Concomitant administration of mefloquine and other related antimalarial compounds (eg, quinine, quinidine and chloroquine) may produce electrocardiographic abnormalities and increase the risk of convulsions (see WARNINGS). If these drugs are to be used in the initial treatment of severe malaria, mefloquine administration should be delayed at least 12 hours after the last dose. Clinically significant QTc prolongation has not been found with mefloquine alone.
-
Ketoconazole (Potent Inhibitor of CYP3A4)
Co-administration of a single 500 mg oral dose of mefloquine with 400 mg of ketoconazole once daily for 10 days in 8 healthy volunteers resulted in an increase in the mean C maxand AUC of mefloquine by 64% and 79%, respectively, and an increase in the mean elimination half-life of mefloquine from 322 hours to 448 hours. Ketoconazole should not be administered with mefloquine or within 15 weeks of the last dose of mefloquine due to the risk of a potentially fatal prolongation of the QTc interval (see WARNINGS).
-
Other Drugs that Prolong the QTc Interval
Co-administration of other drugs known to alter cardiac conduction (eg, anti-arrhythmic or beta-adrenergic blocking agents, calcium channel blockers, antihistamines or H 1-blocking agents, tricyclic antidepressants and phenothiazines) might also contribute to a prolongation of the QTc interval. There are no data that conclusively establish whether the concomitant administration of mefloquine and the above listed agents has an effect on cardiac function.
-
Anticonvulsants
In patients taking an anticonvulsant (eg, valproic acid, carbamazepine, phenobarbital or phenytoin), the concomitant use of mefloquine may reduce seizure control by lowering the plasma levels of the anticonvulsant. Therefore, patients concurrently taking anti-seizure medication and mefloquine should have the blood level of their anti-seizure medication monitored and the dosage adjusted appropriately (see PRECAUTIONS).
- Vaccines
-
Rifampin (Potent Inducer of CYP3A4)
Co-administration of a single 500 mg oral dose of mefloquine and 600 mg of rifampin once daily for 7 days in 7 healthy Thai volunteers resulted in a decrease in the mean C maxand AUC of mefloquine by 19% and 68%, respectively, and a decrease in the mean elimination half-life of mefloquine from 305 hours to 113 hours. Rifampin should be used cautiously in patients taking mefloquine.
-
Inhibitors and Inducers of CYP3A4
Mefloquine does not inhibit or induce the CYP 450 enzyme system. Thus, concomitant administration of mefloquine hydrochloride tablets and substrates of the CYP 450 enzyme system is not expected to result in a drug interaction. However, mefloquine is metablolized by CYP3A4 and inhibitors of CYP3A4 may modify the pharmacokinetics/metabolism of mefloquine, leading to an increase in mefloquine plasma concentrations and potential risk of adverse reactions. Therefore, mefloquine hydrochloride tablets should be used with caution when administered concomitantly with CYP3A4 inhibitors. Similarly, inducers of CYP3A4 may modify the pharmacokinetics/metabolism of mefloquine, leading to a decrease in mefloquine plasma concentrations and potential reduction in efficacy of mefloquine hydrochloride tablets, Therefore, mefloquine hydrochloride tablets should also be used with caution when administered comcomitantly with CYP3A4 inducers.
-
Substrates and Inhibitors of P-glycoprotein
It has been shown in vitrothat mefloquine is a substrate and an inhibitor of P-glycoprotein. Therefore, drug-drug interactions could also occur with drugs that are substrates or are known to modify the expression of this transporter. The clinical relevance of these interactions is not known to date.
-
Other Potential Interactions
No other drug interactions are known. Nevertheless, the effects of mefloquine on travelers receiving concomitant medications, particularly diabetics or patients using anticoagulants, should be checked before departure.
In clinical trials, the concomitant administration of sulfadoxine and pyrimethamine did not alter the adverse reaction profile of mefloquine.
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenesis
-
Mutagenesis
The mutagenic potential of mefloquine was studied in a variety of assay systems including: Ames test, a host-mediated assay in mice, fluctuation tests and a mouse micronucleus assay. Several of these assays were performed with and without prior metabolic activation. In no instance was evidence obtained for the mutagenicity of mefloquine.
-
Impairment of Fertility
Fertility studies in rats at doses of 5, 20, and 50 mg/kg/day of mefloquine have demonstrated adverse effects on fertility in the male at the high dose of 50 mg/kg/day, and in the female at doses of 20 and 50 mg/kg/day. Histopathological lesions were noted in the epididymides from male rats at doses of 20 and 50 mg/kg/day. Administration of 250 mg/week of mefloquine (base) in adult males for 22 weeks failed to reveal any deleterious effects on human spermatozoa.
- Pregnancy
-
Teratogenic Effects
Pregnancy Category B
Data from published studies in pregnant women have shown no increase in the risk of teratogenic effects or adverse pregnancy outcomes following mefloquine treatment or prophylaxis during pregnancy. Reproduction studies in mice, rats and rabbits have shown teratogenic effects at doses similar to the clinical acute treatment dose in humans. Because the studies in humans cannot rule out the possibility of harm, mefloquine should be used during pregnancy only if clearly needed.
Published data on mefloquine use during pregnancy include randomized controlled trials, intervention trials, prospective and retrospective cohort studies, and case series. These data showed that pregnant women who took mefloquine at various doses for both prevention and treatment of malaria did not have an increased risk of teratogenic effects or adverse pregnancy outcomes compared to the background rate in the general population. These data include more than 700 exposures to mefloquine in the first trimester of pregnancy and over 2,000 exposures in the second and third trimester.
Mefloquine administered to pregnant mice, rats, and rabbits was teratogenic at doses similar to the clinical acute treatment dose of 21 to 25 mg/kg, based on body surface area comparisons. In all three animal species, CNS effects (e.g., exencephaly, hydrocephaly or partially missing medulla oblongata) and craniofacial malformations were observed. At the same doses, mefloquine was also embryotoxic in mice and rabbits. All of these findings were observed at doses that were maternally toxic.
-
Nursing Mothers
Mefloquine is excreted in human milk in small amounts, the activity of which is unknown. Based on a study in a few subjects, low concentrations (3% to 4%) of mefloquine were excreted in human milk following a dose equivalent to 250 mg of the free base. Caution should be exercised when administered to a nursing woman.
-
Pediatric Use
Use of mefloquine to treat acute, uncomplicated P. falciparummalaria in pediatric patients is supported by evidence from adequate and well-controlled studies of mefloquine in adults with additional data from published open-label and comparative trials using mefloquine to treat malaria caused by P. falciparumin patients younger than 16 years of age. The safety and effectiveness of mefloquine for the treatment of malaria in pediatric patients below the age of 6 months have not been established.
In several studies, the administration of mefloquine for the treatment of malaria was associated with early vomiting in pediatric patients. Early vomiting was cited in some reports as a possible cause of treatment failure. If a second dose is not tolerated, the patient should be monitored closely and alternative malaria treatment considered if improvement is not observed within a reasonable period of time (see WARNINGSand DOSAGE AND ADMINISTRATION).
-
Geriatric Use
Clinical studies of mefloquine did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Since electrocardiographic abnormalities have been observed in individuals treated with mefloquine (see PRECAUTIONS) and underlying cardiac disease is more prevalent in elderly than in younger patients, the benefits of mefloquine therapy should be weighed against the possibility of adverse cardiac effects in elderly patients.
- ADVERSE REACTIONS
-
Clinical
At the doses used for treatment of acute malaria infections, the symptoms possibly attributable to drug administration cannot be distinguished from those symptoms usually attributable to the disease itself.
Among subjects who received mefloquine for prophylaxis of malaria, the most frequently observed adverse experience was vomiting (3%). Dizziness, syncope, extrasystoles and other complaints affecting less than 1% were also reported.
Two serious adverse reactions were cardiopulmonary arrest in one patient shortly after ingesting a single prophylactic dose of mefloquine while concomitantly using propranolol (see PRECAUTIONS: Drug Interactions), and encephalopathy of unknown etiology during prophylactic mefloquine administration. The relationship of encephalopathy to drug administration could not be clearly established.
Among subjects who received mefloquine for treatment, the most frequently observed adverse experiences included: dizziness, myalgia, nausea, fever, headache, vomiting, chills, diarrhea, skin rash, abdominal pain, fatigue, loss of appetite, and tinnitus. Those side effects occurring in less than 1% included bradycardia, hair loss, emotional problems, pruritus, asthenia, transient emotional disturbances and telogen effluvium (loss of resting hair). Seizures have also been reported.
-
Laboratory
The most frequently observed laboratory alterations which could be possibly attributable to drug administration were decreased hematocrit, transient elevation of transaminases, leukopenia and thrombocytopenia. These alterations were observed in patients with acute malaria who received treatment doses of the drug and were attributed to the disease itself.
During prophylactic administration of mefloquine to indigenous populations in malaria-endemic areas, the following alterations in laboratory values were observed: transient elevation of transaminases, leukocytosis or thrombocytopenia.
Because of the long half-life of mefloquine, adverse reactions to mefloquine may occur or persist up to several weeks after discontinuation of the drug.
-
Postmarketing
Postmarketing surveillance indicates that the same kind of adverse reactions are reported during prophylaxis, as well as acute treatment. Because these adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to mefloquine exposure.
The most frequently reported adverse reactions are nausea, vomiting, loose stools or diarrhea, abdominal pain, dizziness or vertigo, loss of balance, and neuropsychiatric events such as headache, somnolence, and sleep disorders (insomnia, abnormal dreams). These adverse reactions may occur early in the course of mefloquine use. It has been reported that dizziness or vertigo, tinnitus and hearing impairment, and loss of balance may continue for months or years after discontinuation of the drug and may be permanent in some cases.
More severe neuropsychiatric disorders have been reported such as: sensory and motor neuropathies (including paresthesia, tremor and ataxia), convulsions, agitation or restlessness, anxiety, depression, mood swings, panic attacks, memory impairment, confusion, hallucinations, aggression, psychotic or paranoid reactions and encephalopathy. Cases of suicidal ideation and suicide have been reported.
Other less frequently reported adverse reactions include:
Cardiovascular Disorders
circulatory disturbances (hypotension, hypertension, flushing, syncope), chest pain, tachycardia or palpitation, bradycardia, irregular heart rate, extrasystoles, A-V block, and other transient cardiac conduction alterations.
Skin Disorders
rash, exanthema, erythema, urticaria, pruritus, edema, hair loss, erythema multiforme, and Stevens-Johnson syndrome.
Musculoskeletal Disorders
muscle weakness, muscle cramps, myalgia, and arthralgia.
Respiratory Disorders
dyspnea, pneumonitis of possible allergic etiology
Hepatobiliary Disorders
drug-related hepatic disorders from asymptomatic transient transaminase elevations to hepatic failure
Blood and Lymphatic System Disorders
agranulocytosis, aplastic anemia
Ocular Disorders
visual impairment, vision blurred, cataracts, retinal disorders, optic neuropathy
Other Symptoms
asthenia, malaise, fatigue, fever, hyperhidrosis, chills, dyspepsia and loss of appetite.
- OVERDOSAGE
-
Symptoms and Signs
In cases of overdosage with mefloquine, the symptoms mentioned under ADVERSE REACTIONSmay be more pronounced.
- Treatment
- DOSAGE AND ADMINISTRATION
-
Malaria Treatment in Adults
Treatment of mild to moderate malaria in adults caused by mefloquine-susceptible strains of P. falciparum or by P. vivax
Dosage: Five tablets (1250 mg) mefloquine hydrochloride to be given as a single oral dose. The drug should not be taken on an empty stomach and should be administered with at least 8 oz (240 mL) of water.
If a full-treatment course with mefloquine does not lead to improvement within 48 to 72 hours, mefloquine should not be used for retreatment. An alternative therapy should be used. Similarly, if previous prophylaxis with mefloquine failed, mefloquine should not be used for curative treatment (see INDICATIONS AND USAGE).
Note:Patients with acute P. vivaxmalaria, treated with mefloquine, are at high risk of relapse because mefloquine does not eliminate exoerythrocytic (hepatic phase) parasites. To avoid relapse after initial treatment of the acute infection with mefloquine, patients should subsequently be treated with an 8-aminoquinoline derivative (eg, primaquine).
-
Malaria Prophylaxis in Adults
Dosage: One 250 mg mefloquine hydrochloride tablet once weekly.
Prophylactic drug administration should begin 1 week before arrival in an endemic area. Subsequent weekly doses should be taken regularly, always on the same day of each week, preferably after the main meal. To reduce the risk of malaria after leaving an endemic area, prophylaxis must be continued for 4 additional weeks to ensure suppressive blood levels of the drug when merozoites emerge from the liver. Tablets should not be taken on an empty stomach and should be administered with at least 8 oz (240 mL) of water.
In certain cases, eg, when a traveler is taking other medication, it may be desirable to start prophylaxis 2 to 3 weeks prior to departure, in order to ensure that the combination of drugs is well tolerated (see PRECAUTIONS: Drug Interactions).
When prophylaxis with mefloquine fails, physicians should carefully evaluate which antimalarial to use for therapy.
-
Malaria Treatment in Pediatric Patients
Treatment of mild to moderate malaria in pediatric patients caused by mefloquine-susceptible strains of P. falciparum
Dosage: 20 to 25 mg/kg body weight. Splitting the total therapeutic dose into 2 doses taken 6 to 8 hours apart may reduce the occurrence or severity of adverse effects. The pediatric dose should not exceed the adult dose.
Experience with mefloquine in pediatric patients weighing less than 20 kg is limited. The drug should not be taken on an empty stomach and should be administered with ample water. The tablets may be crushed and suspended in a small amount of water, milk or other beverage for administration to small children and other persons unable to swallow them whole.
If a full-treatment course with mefloquine does not lead to improvement within 48 to 72 hours, mefloquine should not be used for retreatment. An alternative therapy should be used. Similarly, if previous prophylaxis with mefloquine has failed, mefloquine should not be used for curative treatment.
In pediatric patients, the administration of mefloquine for the treatment of malaria has been associated with early vomiting. In some cases, early vomiting has been cited as a possible cause of treatment failure (see PRECAUTIONS). If a significant loss of drug product is observed or suspected because of vomiting, a second full dose of mefloquine should be administered to patients who vomit less than 30 minutes after receiving the drug. If vomiting occurs 30 to 60 minutes after a dose, an additional half-dose should be given. If vomiting recurs, the patient should be monitored closely and alternative malaria treatment considered if improvement is not observed within a reasonable period of time.
The safety and effectiveness of mefloquine to treat malaria in pediatric patients below the age of 6 months have not been established.
-
Malaria Prophylaxis in Pediatric Patients
The recommended prophylactic dose of mefloquine is approximately 5 mg/kg body weight once weekly. One 250 mg mefloquine hydrochloride tablet should be taken once weekly in pediatric patients weighing over 45 kg. In pediatric patients weighing less than 45 kg, the weekly dose decreases in proportion to body weight:
30 to 45 kg: 3/4 tablet
20 to 30 kg: 1/2 tablet
Experience with mefloquine in pediatric patients weighing less than 20 kg is limited.
-
HOW SUPPLIED
Mefloquine hydrochloride tablets USP, 250 mg are round, white to off white tablets, scored, debossed GP 118 on one side and plain on the reverse side, and are supplied as follows:
Bottle of 5 tablets – NDC: 62135-973-11
Bottle of 25 tablets – NDC: 62135-973-25
Dispense in tight, light-resistant container as determined in the USP using a child-resistant closure.
Store at 20°-25°C (68°-77°F) (see USP Controlled Room Temperature).
-
ANIMAL TOXICOLOGY
Ocular lesions were observed in rats fed mefloquine daily for 2 years. All surviving rats given 30 mg/kg/day had ocular lesions in both eyes characterized by retinal degeneration, opacity of the lens, and retinal edema. Similar but less severe lesions were observed in 80% of female and 22% of male rats fed 12.5 mg/kg/day for 2 years. At doses of 5 mg/kg/day, only corneal lesions were observed. They occurred in 9% of rats studied.
Male Wistar rats orally administered-mefloquine daily for 22 days at the equivalent human therapeutic plasma concentration showed CNS penetration of mefloquine, with a 30-50 fold greater brain/plasma drug ratio up to 10 days after the final dose administered.1
-
REFERENCES
- Baudry S., Pham YT., Baune B., Vidrequin S., Crevoisier CH., Gimenez F., Fainotti R. (1997). Stereoselective passage of mefloquine through the blood brain barrier in the rat. J. Pharm. Pharmacol. 49: 1086-1090.
Manufactured for:
Chartwell RX, LLC.
Congers, NY 10920
L72467
Revised: 11/2024
Pharmacist: Dispense Medication Guide and Wallet Card to each patient.
Print Medication Guides at: www.chartwellpharma.com/medguides.
Print Wallet Cards at www.chartwellpharma.com/our-products/
- Baudry S., Pham YT., Baune B., Vidrequin S., Crevoisier CH., Gimenez F., Fainotti R. (1997). Stereoselective passage of mefloquine through the blood brain barrier in the rat. J. Pharm. Pharmacol. 49: 1086-1090.
-
MEDICATION GUIDE
Print Medication Guides at: www.chartwellpharma.com/medguides.
MEDICATION GUIDE
Mefloquine Hydrochloride Tablets, USPImportant:
Your doctor or pharmacist will give you an Information Wallet Card along with this Medication Guide.It has important information about mefloquine and should be carried with you at all times while you take mefloquine.
What is the most important information I should know about mefloquine?
Mefloquine can cause serious side effects, including:
1. Heart Problems.
Do not take halofantrine (used to treat malaria) or ketoconazole (used for fungal infections) with mefloquine or within 15 weeks of your last dose of mefloquine. You may get serious heart problems (problems with the electrical system of your heart called QT prolongation) that can lead to death. Do not take quinine (Qualaquin) or quinidine (used to treat malaria or irregular heart beat) with mefloquine. You may get serious heart problems.
2. Mental problems.Symptoms of serious mental problems may include:
- severe anxiety
- paranoia (feelings of mistrust towards others)
- hallucinations (seeing or hearing things that are not there)
- depression
- feeling restless
- unusual behavior
- feeling confused
Some people who take mefloquine think about suicide (putting an end to their life). Some people who were taking mefloquine committed suicide. It is not known if mefloquine was responsible for those suicides.
If you have any of these serious mental problems, or you develop other serious side effects or mental problems, you should contact your doctor right away as it may be necessary to stop taking mefloquine and use a different medicine to prevent malaria.
3. Problems with your body’s nervous system.Symptoms of serious nervous system problems may include:
- dizziness
- a feeling that you or things around you are moving or spinning (vertigo)
- loss of balance
- ringing sound in your ears (tinnitus)
- convulsions (seizures) in people who already have seizures (epilepsy)
- convulsions (seizures) in people who take quinine or chloroquine (used to treat malaria) with mefloquine. Do not take quinine (Qualaquin) or chloroquine (Aralen) with mefloquine.
- unable to sleep (insomnia)
Dizziness, vertigo, tinnitus, and loss of balance can go on for months or years after mefloquine is stopped or may become permanent in some people.
Important:
You need to take malaria prevention medicine before you travel to a malaria area, while you are in a malaria area, and after you return from a malaria area.
- If you are told by a doctor to stop taking mefloquine because of the side effects or for other reasons, you will need to take different malaria medicine.
- If you do not have access to a doctor or to another medicine and have to stop taking mefloquine, leave the malaria area and contact a doctor as soon as possible because leaving the malaria area may not protect you from getting malaria. You will still need to take a malaria prevention medicine for another 4 weeks after you leave the malaria area.
What is Mefloquine?
Mefloquine is a prescription medicine used to prevent and treat malaria. Malaria can be a life-threatening infection. Mefloquine does not work for all types of malaria.
It is not known if mefloquine is safe and effective in children under 6 months old for the treatment of malaria. It is not known how well mefloquine works to prevent malaria in children weighing less than 44 pounds (20 kilograms).
Who should not take Mefloquine?
Do not take Mefloquine if you have:
- depression or had depression recently
- had recent mental problems, including anxiety disorder, schizophrenia, or psychosis (losing touch with reality)
- seizures or had seizures (epilepsy or convulsions)
- an allergy to quinine, quinidine, mefloquine or any ingredients in mefloquine. See the end of this Medication Guide for a complete list of ingredients in mefloquine.
Talk to your doctor before you take mefloquine if you have any of the medical conditions listed above.
What should I tell my doctor before taking mefloquine?
Before taking mefloquine, tell your doctor about all your medical conditions, including if you have:
- heart disease
- liver problems
- seizures or epilepsy
- diabetes
- blood clotting problems or take blood thinner medicines (anticoagulants)
- mental problems
- are pregnant or plan to become pregnant. It is not known if mefloquine will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- You should use birth control while you take mefloquine and for 3 months after you stop mefloquine.If you have an unplanned pregnancy, talk to your doctor right away.
- are breast-feeding or plan to breast-feed. Mefloquine can pass into your milk and may harm your baby. Ask your doctor if you will need to stop breast-feeding or use a different medicine.
Contact your doctor right away if you have a fever after leaving a malaria area.
Tell your doctor about all the medicines you take,including prescription and nonprescription medicines, vitamins, and herbal supplements. Mefloquine and other medicines may affect each other causing side effects.
How should I take Mefloquine?
- Take mefloquine exactly as your doctor tells you to take it. Your doctor will tell you how many mefloquine tablets to take and when to take them.
- You will start taking mefloquine to prevent malaria between 1 to 3 weeks before you travel to a malaria area.
- Take mefloquine just after eating your largest meal of the day and with at least 1 cup (8 ounces) of water.
- Do not take mefloquine on an empty stomach.
- If you vomit after taking mefloquine, contact your doctor to see if you should take another dose.
- Continue taking mefloquine for 4 weeks after returning from a malaria area .
- Mefloquine tablets may be crushed and mixed with a small amount of water, milk or other beverage for children or other people unable to swallow mefloquine whole. Your doctor will tell you the correct dose for your child based on your child’s weight.
- If you take Mefloquine for a year or longer, your doctor should check your
- eyes (especially if you have trouble seeing while you take mefloquine)
- liver function (to see if there has been damage to your liver)
- Use protective clothing, insect repellents, and bednets to protect you from being bitten by mosquitoes. Medicine alone does not always stop you from catching malaria from mosquito bites.
What should I avoid while taking mefloquine?
Avoid activities such as driving a car or using heavy machinery or other activities needing alertness and careful movements (fine motor coordination) until you know how mefloquine affects you. You may feel dizzy or lose your balance. This could happen for months or years after you stop taking mefloquine and can be permanent in some cases. See “What are the possible side effects of mefloquine?”
What are the possible side effects of mefloquine?
See “What is the most important information I should know about mefloquine?”
Mefloquine may cause serious side effects, including:
- liver problems
Call your healthcare provider right away if you have unexplained symptoms such as nausea or vomiting, stomach pain, fever, weakness, itching, unusual tiredness, loss of appetite, light colored bowel movements, dark colored urine, yellowing of your skin or the white of your eyes.
The most common side effects of mefloquine include:
- nausea
- vomiting
- diarrhea
- abdominal pain
- headache
The most common side effects in people who take mefloquine for treatment include:
- muscle pain
- fever
- chills
- skin rash
- fatigue
- loss of appetite
- irregular heart beat
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of mefloquine. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store mefloquine?
- Store mefloquine at room temperature between 68ºF to 77ºF (20ºC to 25ºC)
- Safely throw away medicine that is out of date or no longer needed.
Keep mefloquine and all medicines out of the reach of children.
General information about the safe and effective use of mefloquine.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use mefloquine for a condition for which it was not prescribed. Do not give mefloquine to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about mefloquine. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about mefloquine that is written for health professionals.
What are the ingredients in mefloquine?
Active ingredients: mefloquine hydrochloride
Inactive ingredients: crospovidone, lactose monohydrate, low-substituted hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose, pregelatinized starch and talc.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured for :
Chartwell RX, LLC
Congers, NY 10920
L72468
Revised : 11/2024
-
SPL UNCLASSIFIED SECTION
Print Wallet Cards at www.chartwellpharma.com/our-products/
Information Wallet Card:
Mefloquine Hydrochloride Tablets, USP
It is important that you read the entire Medication Guide for additional information on mefloquine.
Carry this wallet card with you when you are taking mefloquine.
Important: You need to take malaria prevention medicine before you travel to a malaria area, while you are in a malaria area, and after you return from a malaria area.
Mefloquine can cause serious side effects, including:
1. Heart problems.
Do nottake halofantrine (used to treat malaria) or ketoconazole (used for fungal infections) with mefloquine or within 15 weeks of your last dose of mefloquine. You may get serious heart problems that can lead to death. Do nottake quinine (Qualaquin) or quinidine (used to treat malaria or irregular heart beat) with mefloquine. You may get serious heart problems. Mefloquine may cause serious problems with the electrical system of your heart, called QT prolongation.
2. Mental problems.Symptoms of serious mental problems may include severe anxiety, paranoia (feelings of mistrust towards others), hallucinations (seeing or hearing things that are not there), depression, feeling restless, unusual behavior or feeling confused. Some people who take mefloquine think about suicide (putting an end to their life). Some people who were taking mefloquine committed suicide. It is not known if mefloquine was responsible for those suicides.
If you have any of these serious mental problems you should contact your doctor right away as it may be necessary to stop taking mefloquine and use a different medicine to prevent malaria.
3. Problems with your body’s nervous system.
Do nottake quinine (Qualaquin) or chloroquine (Aralen) (used to treat malaria) with mefloquine. You may have a greater risk for convulsions (seizures).
Symptoms of serious nervous system problems may include dizziness, a feeling that you or things around you are moving or spinning (vertigo), loss of balance, ringing in your ears (tinnitus), convulsions (seizures) in people who already have seizures, or you are unable to sleep (insomnia).
These serious mental and nervous system side effects can go on for months or years after mefloquine is stopped or may become permanent in some people.
If you are told by a doctor to stop taking mefloquine because of the side effects or for other reasons, you will need to take a different malaria medicine.
If you do not have access to a doctor or to a different medicine and have to stop taking mefloquine, leave the malaria area and contact a doctor as soon as possible because leaving the malaria area may not protect you from getting malaria. You will still need to take a malaria prevention medicine for another 4 weeks after you leave the malaria area.
Mefloquine may cause serious liver problems. Symptoms of liver problems include nausea, vomiting, loss of appetite, unusual tiredness, stomach pain, fever, weakness, itching, light-colored bowel movements, dark colored urine, yellowing of your skin or the white of your eyes. The most common side effectsof mefloquine include nausea, vomiting, diarrhea, abdominal pain and headache.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of mefloquine. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What should I avoid while taking mefloquine?
Avoid activities such as driving a car or using heavy machinery or other activities needing alertness and careful movements (fine motor coordination) until you know how mefloquine affects you. You may feel dizzy or lose your balance. This could happen for months or years after you stop taking mefloquine and can be permanent in some cases.
Manufactured for :
Chartwell RX, LLC
Congers, NY 10920
L72471
Revised : 11/2024
-
PRINCIPAL DISPLAY PANEL
Mefloquine Hydrochloride Tablets, USP 250 mg - NDC: 62135-973-11 - 5s Bottle Label

Mefloquine Hydrochloride Tablets, USP 250 mg - NDC: 62135-973-25 - 25s Bottle Label

-
INGREDIENTS AND APPEARANCE
MEFLOQUINE HYDROCHLORIDE
mefloquine hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62135-973 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MEFLOQUINE HYDROCHLORIDE (UNII: 5Y9L3636O3) (MEFLOQUINE - UNII:TML814419R) MEFLOQUINE HYDROCHLORIDE 250 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSPOVIDONE (120 .MU.M) (UNII: 68401960MK) HYDROXYPROPYL CELLULOSE, LOW SUBSTITUTED (UNII: 2165RE0K14) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color white ((to off white)) Score 2 pieces Shape ROUND Size 11mm Flavor Imprint Code GP;118 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62135-973-25 25 in 1 BOTTLE; Type 0: Not a Combination Product 11/15/2024 2 NDC: 62135-973-11 5 in 1 BOTTLE; Type 0: Not a Combination Product 11/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076175 02/20/2022 Labeler - Chartwell RX, LLC (079394054)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.