HEMOFIL M- antihemophilic factor human kit
HEMOFIL M by
Drug Labeling and Warnings
HEMOFIL M by is a Other medication manufactured, distributed, or labeled by Takeda Pharmaceuticals America, Inc., BAXALTA US Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- HEMOFIL M
-
DESCRIPTION
HEMOFIL M, Antihemophilic Factor (Human) (AHF), Method M, Monoclonal Purified, is a sterile, nonpyrogenic, dried preparation of antihemophilic factor (Factor VIII, Factor VIII:C, AHF) in concentrated form with a specific activity range of 2 to 22 AHF International Units/mg of total protein. HEMOFIL M contains a maximum of 12.5 mg/mL Albumin, and per AHF International Unit, 0.07 mg polyethylene glycol (3350), 0.39 mg histidine as stabilizing agents, not more than 0.1 mg glycine, 0.1 ng mouse protein, 18 ng organic solvent (tri-n-butyl phosphate) and 50 ng detergent (octoxynol 9). In the absence of the added Albumin (Human), the specific activity is approximately 2,000 AHF International Units/mg of protein [see Clinical Pharmacology].
HEMOFIL M is prepared by the Method M process from pooled human plasma by immunoaffinity chromatography utilizing a murine monoclonal antibody to Factor VIII:C, followed by an ion exchange chromatography step for further purification. Source material may be provided by other US licensed manufacturers. HEMOFIL M also includes an organic solvent (tri-n-butyl phosphate) and detergent (octoxynol 9) virus inactivation step designed to reduce the risk of transmission of hepatitis and other viral diseases. The process further includes a nanofiltration step between immunoaffinity chromatography and ion-exchange chromatography as an additional viral clearance step to further improve the viral safety margin of the final product.
Use of an organic solvent (tri-n-butyl phosphate; TNBP) in the manufacture of Antihemophilic Factor (Human) has little or no effect on AHF activity, while lipid enveloped viruses, such as hepatitis B and human immunodeficiency virus (HIV) would be inactivated.1 The nanofiltration step integrated into the manufacture of AHF-M further enhances the safety margin with respect to adventitious viruses. Each bottle of HEMOFIL M is labeled with the AHF activity expressed in International Units (IU) per bottle. This potency assignment is referenced to the World Health Organization International Standard. The purity of HEMOFIL M has been thought to influence the difficulty of producing an accurate potency measurement. Experiments have shown that to achieve accurate activity levels, such a potency assay should be conducted using plastic test tubes and pipets as well as substrate containing normal levels of von Willebrand's Factor.
In vitro studies demonstrate that the HEMOFIL M manufacturing process provides for viral reduction. These reductions are achieved through a combination of process chemistry, partitioning and/or inactivation during solvent/detergent treatment, and immunoaffinity chromatography. Introduction of a nanofiltration step with the 0.1µm prefilter and the ASAHI Planova 20N nanofilter provides a virus removal capacity for human immunodeficiency virus, Type 1 (HIV-1), hepatitis A virus (HAV), bovine viral diarrhea virus (BVDV), pseudorabies virus (PRV), mice minute virus (MMV), and human parvovirus B19 (B19V) in the order of four (4) logs or higher. B19V removal data were obtained with a Polymerase Chain Reaction (PCR) assay not correlated to an infectivity assay.
Studies for nanofiltration and other process steps, summarized in Table 1, demonstrate virus clearance during the HEMOFIL M manufacturing process using HIV-1; BVDV, a generic model for lipid enveloped RNA viruses, such as hepatitis C virus (HCV); PRV, a model for lipid enveloped DNA viruses, such as hepatitis B virus (HBV); canine parvovirus (CPV), a model for non-lipid enveloped DNA viruses, such as B19V, HAV, and MMV.
Table 1 In Vitro Virus Clearance During the Manufacture of HEMOFIL M NT not tested - * As Solvent/Detergent treatment does not inactivate non-lipid enveloped viruses.
- † Not Applicable for lipid enveloped viruses due to the presence of (virucidal) solvent/detergent reagents in the starting material.
Process Step Evaluated
Virus Clearance, log 10
Lipid-enveloped
Non-Lipid enveloped
HIV-1
BVDV
PRV
HAV
CPV
MMV
Solvent/Detergent Treatment
>4.8
>6.8
>6.9
NT*
NT*
NT*
Immunoaffinity Chromatography
N.A.†
N.A.†
N.A.†
≥4.5
≥3.9
NT
Nanofiltration
>5.5
>4.6
>4.4
>5.4
NT
>5.0
Cumulative Total, log10
>10.3
>11.4
>11.3
>9.9
≥3.9
>5.0
-
CLINICAL PHARMACOLOGY
Antihemophilic factor (AHF) is a protein found in normal plasma which is necessary for clot formation. The administration of HEMOFIL M provides an increase in plasma levels of AHF and can temporarily correct the coagulation defect of patients with hemophilia A (classical hemophilia). The half-life of HEMOFIL M administered to Factor VIII deficient patients has been shown to be 14.8 ± 3.0 hours.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Hypersensitivity
Allergic-type hypersensitivity reactions, including anaphylaxis, have been reported with HEMOFIL M and have been manifested by bronchospasm, dyspnea, hypotension, chest pain, facial edema, urticaria, rash, flushing, pruritus, and nausea.
Neutralizing Antibodies
The development of neutralizing antibodies (inhibitors) to Factor VIII is a known complication of the treatment of patients with Hemophilia A. Inhibitors have predominantly been reported in previously untreated patients. The risk of developing inhibitors is correlated to the extent of exposure to Factor VIII, the risk being highest within the first 20 exposure days, and to other genetic and environmental factors. The risk for inhibitor development depends on a number of factors relating to the characteristics of the patient (e.g., type of the Factor VIII gene mutation, family history, ethnicity), which are believed to represent the most significant risk factors for inhibitor formation.
Transmission of Infectious Agents
Because HEMOFIL M is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. This also applies to unknown or emerging viruses and other pathogens.
All infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Baxalta US Inc. at 1-800-423-2090 (in the U.S.) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. The physician should discuss the risks and benefits of this product with the patient.
Individuals who receive infusions of blood or plasma products may develop signs and/or symptoms of some viral infections, particularly non-A, non-B hepatitis. As indicated under Clinical Pharmacology, however, a group of such patients treated with HEMOFIL M did not demonstrate signs or symptoms of non-A, non-B hepatitis over observation periods ranging from three to nine months.
-
PRECAUTIONS
Identification of the clotting defect as a Factor VIII deficiency is essential before the administration of HEMOFIL M is initiated.
Factor VIII Inhibitors
Evaluate patients for the development of Factor VIII inhibitors if the expected plasma Factor VIII activity levels are not attained or if bleeding is not controlled with an appropriate dose.
No benefit may be expected from this product in treating other deficiencies.
Formation of Antibodies to Mouse Protein
HEMOFIL M contains trace amounts of mouse protein (less than 0.1 ng/AHF activity units). The possibility exists that patients treated with HEMOFIL M may develop hypersensitivity to the mouse proteins. There have been no cases of hypersensitivity to the mouse proteins reported.
Increase in Pulse Rate
Determine the pulse rate before and during administration of HEMOFIL M. Should a significant increase occur, reduce the rate of administration or temporarily halt the injection to allow the symptoms to disappear promptly.
Laboratory Tests
Perform appropriate laboratory tests on the patient's plasma at suitable intervals to ensure that adequate AHF levels have been reached and are maintained.
If the AHF content of the patient's plasma fails to reach expected levels or if bleeding is not controlled after apparently adequate dosage, the presence of inhibitor should be suspected. By appropriate laboratory procedures, the presence of inhibitor can be demonstrated and quantified in terms of AHF units neutralized by each mL of plasma or by the total estimated plasma volume.
If the inhibitor is at low levels (i.e., <10 Bethesda units per mL), after administration of sufficient AHF units to neutralize the inhibitor, additional AHF units will elicit the predicted response.
Pregnancy
Animal reproduction studies have not been conducted with HEMOFIL M. The safety of HEMOFIL M for use in pregnant women has not been established. It is not known whether HEMOFIL M can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. HEMOFIL M should be given to a pregnant woman only if clearly needed.
Nursing Mothers
The safety of HEMOFIL M for use in nursing mothers has not been established. It is not known whether this drug is excreted into human milk. Physicians should carefully consider the potential risks and benefits for each specific patient before prescribing HEMOFIL M. HEMOFIL M should be given to nursing mothers only if clinically indicated.
-
ADVERSE REACTIONS
Adverse Reactions from Clinical Trials
The adverse reactions presented in this section have been identified based on clinical trial experience with HEMOFIL M in patients previously treated with other Factor VIII concentrates or blood products (N = 74), and previously untreated patients (PUPs; N = 50).
- * In a study that included 43 evaluable PUPs and 10 minimally treated patients (MTPs), i.e., patients with a single exposure to other Factor VIII concentrates or blood products, 3 of the total of 53 patients (5.7%) developed an inhibitor while on study.
Clinical Trial Adverse Reactions
System Organ Class (SOC)
Preferred MedDRA Term
Number of Cases
(Frequency
Percentage)
BLOOD AND LYMPHATIC
SYSTEM DISORDERS
Factor VIII inhibition
3 (5.7%)*
NERVOUS SYSTEM DISORDERS
Dizziness
1 (0.8%)
Headache
1 (0.8%)
Dysgeusia
1 (0.8%)
GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS
Pyrexia
1 (0.8%)
Infusion site inflammation
2 (1.6%)
HEMOFIL M was administered to 11 patients previously untreated with Antihemophilic Factor (Human). They have shown no signs of hepatitis or HIV infection following three to nine months of evaluation.
A study of 25 patients treated with HEMOFIL M and monitored for three to six months has demonstrated no evidence of antibody response to mouse protein. More than 1,000 infusions of HEMOFIL M have been administered during the clinical trials. Reported events included a single episode each of chest tightness, fuzziness and dizziness, and one patient reported an unusual taste after each infusion.
Post-marketing Adverse Reactions
In addition to clinical trials, the following adverse reactions have been reported in the post-marketing experience, listed by MedDRA System Organ Class (SOC), then by Preferred Term.
Immune System Disorders: anaphylactic reaction, hypersensitivity
Eye Disorders: visual impairment, ocular hyperemia
Cardiac Disorders: cyanosis, bradycardia, tachycardia
Vascular Disorders: hypotension, flushing
Respiratory, Thoracic, and Mediastinal Disorders: bronchospasm, dyspnea, cough, hyperventilation
Gastrointestinal Disorders: diarrhea, vomiting, nausea, abdominal pain
Skin and Subcutaneous Tissue Disorders: urticaria, rash, pruritus, hyperhidrosis
Musculoskeletal and Connective Tissue Disorders: musculoskeletal pain
General Disorders and Administration Site Conditions: facial edema, edema, chills, fatigue, chest pain, irritability
-
DOSAGE AND ADMINISTRATION
For intravenous use only.
The expected in vivo peak AHF level, expressed as IU/dL of plasma or % (percent) of normal, can be calculated by multiplying the dose administered per kg body weight (IU/kg) by two. This calculation is based on the clinical finding by Abildgaard, et al,2 which is supported by data from the collaborative study of in vivo recovery and survival with 15 different lots of HEMOFIL M on 56 hemophiliacs that demonstrated a mean peak recovery point above the mean pre-infusion baseline of about 2.0 IU/dL per infused IU/kg body weight.3
Examples:
- (1) A dose of 1750 IU AHF administered to a 70 kg patient, i.e., 25 IU/kg (1750/70), should be expected to cause a peak post-infusion AHF increase of 25 x 2 = 50 IU/dL (50% of normal).
- (2) A peak level of 70% is required in a 40 kg child. In this situation the dose would be 70/2 x 40 = 1400 IU.
Recommended Dosage Schedule
Physician supervision of the dosage is required. The following dosage schedule may be used as a guide.
HEMORRHAGE
Degree of hemorrhage
Required peak post-infusion AHF activity in the blood (as % of normal or IU/dL plasma)
Frequency of infusion
Early hemarthrosis or muscle bleed or oral bleed
20-40
Begin infusion every 12 to 24 hours for one-three days until the bleeding episode as indicated by pain is resolved or healing is achieved.
More extensive hemarthrosis, muscle bleed, or hematoma
30-60
Repeat infusion every 12 to 24 hours for usually three days or more until pain and disability are resolved.
Life threatening bleeds such as head injury, throat bleed, severe abdominal pain
60-100
Repeat infusion every 8 to 24 hours until threat is resolved.
SURGERY
Type of operation
Minor surgery, including tooth extraction
60-80
A single infusion plus oral antifibrinolytic therapy within one hour is sufficient in approximately 70% of cases.
Major surgery
80-100
(pre- and post-operative)
Repeat infusion every 8 to 24 hours depending on state of healing.
If bleeding is not controlled with the prescribed dose, determine the plasma level of Factor VIII and administer a sufficient dose of HEMOFIL M to achieve a satisfactory clinical response.
Under certain circumstances (e.g., presence of a low titer inhibitor) doses larger than those recommended may be necessary as per standard care. In patients with high titer Factor VIII inhibitors, HEMOFIL M therapy may not be effective and other therapeutic options should be considered. The dosage and duration of treatment depend on the severity of Factor VIII deficiency, the location and extent of the bleeding, and the patient's clinical condition. Careful control of replacement therapy is especially important in cases of major surgery or life threatening hemorrhages.
Reconstitution: Use Aseptic Technique
- 1. Bring HEMOFIL M (dry concentrate) and Sterile Water for Injection, USP, (diluent) to room temperature.
- 2. Remove caps from concentrate and diluent bottles to expose central portion of rubber stoppers.
- 3. Cleanse stoppers with germicidal solution.
- 4. Remove protective covering from one end of double-ended needle and insert exposed needle through diluent stopper.
- 5. Remove protective covering from other end of double-ended needle. Invert diluent bottle over upright HEMOFIL M bottle, then rapidly insert free end of the needle through the HEMOFIL M bottle stopper at its center. The vacuum in the HEMOFIL M bottle will draw in the diluent.
- 6. Disconnect the two bottles by removing needle from diluent bottle stopper, then remove needle from HEMOFIL M bottle. Swirl gently until all material is dissolved. Be sure that HEMOFIL M is completely dissolved; otherwise active material will be removed by the filter.
Note: Do not refrigerate after reconstitution.
Administration: Use Aseptic Technique
- Intravenous administration only.
- Administer at room temperature not more than 3 hours after reconstitution.
- Record the name and batch number of the product in order to maintain a link between the patient and the batch of the product.
Intravenous Syringe Injection
- Visually inspect parenteral product for particulate matter and discoloration prior to administration. The solution should be colorless in appearance. Do not administer if particulate matter or discoloration is found.
- Plastic syringes are recommended for use with this product. The ground glass surface of all-glass syringes tend to stick with solutions of this type.
- 1. Attach filter needle to a disposable syringe and draw back plunger to admit air into syringe.
- 2. Insert needle into reconstituted HEMOFIL M.
- 3. Inject air into bottle and then withdraw the reconstituted material into the syringe.
- 4. Remove and discard the filter needle from the syringe; attach a suitable needle and inject intravenously as instructed under Rate of Administration.
- 5. If a patient is to receive more than one bottle of HEMOFIL M, the contents of two bottles may be drawn into the same syringe by drawing up each bottle through a separate unused filter needle. This practice lessens the loss of HEMOFIL M. Filter needles are intended to filter the contents of a single bottle of HEMOFIL M only.
Rate of Administration
Administer HEMOFIL M at a rate of up to 10 mL per minute. Infuse HEMOFIL M at a rate of administration that ensures the comfort of the patient [see Precautions: Increase of Pulse Rate].
-
HOW SUPPLIED
HEMOFIL M is available as single-dose bottles that contain the following nominal potencies:
Nominal Potency
Kit NDC Number
250 IU
0944-3940-02
500 IU
0944-3942-02
1000 IU
0944-3944-02
1700 IU
0944-3946-02
Each bottle is labeled with the potency in International Units, and is packaged together with 10 mL of Sterile Water for Injection, USP, a double-ended needle, and a filter needle.
Not made with natural rubber latex.
-
Storage
HEMOFIL M can be stored at 2°C to 8°C (36°F to 46°F) or at room temperature, not to exceed 30°C (86°F), until expiration date noted on the package.
Do not freeze.
Information for Patients
- Advise patients to report any adverse reactions or problems following HEMOFIL M administration to their physician or healthcare provider.
- Advise pregnant women or immune compromised individuals of the effects of Parvovirus B19. Symptoms include fever, drowsiness, chills, runny nose followed about two weeks later by a rash, and joint pain.
- Inform patients of the signs and symptoms of hepatitis A, which include several days to weeks of poor appetite, tiredness, and low-grade fever followed by nausea, vomiting, and pain in the belly. Dark urine and a yellowed complexion are also common symptoms. Encourage patients to consult their physician if such symptoms appear.
- Inform patients of the early signs of hypersensitivity reactions including hives, generalized urticaria, facial edema, flushing, nausea, tightness of the chest, wheezing, dyspnea, hypotension, and anaphylaxis. Advise patients to discontinue use of the product and contact their physician if these symptoms occur.
-
REFERENCES
- 1. Horowitz B, Wiebe ME, Lippin A, et al: Inactivation of viruses in labile blood derivatives: 1. Disruption of lipid enveloped viruses by tri(n-butyl)phosphate detergent combinations. Transfusion 25:516-522, 1985.
- 2. Abildgaard CF, Simone JV, Corrigan JJ, et al: Treatment of hemophilia with glycine-precipitated Factor VIII. New Eng J Med 275:471-475, 1966.
- 3. Addiego, Jr. JE, Gomperts E, Liu S. et al: Treatment of hemophilia A with a highly purified Factor VIII concentrate prepared by Anti-FVIIIc immunoaffinity chromatography. Thrombosis and Haemostasis 67:19-27, 1992.
To enroll in the confidential, industry-wide Patient Notification System, call 1-888-873-2838.
BAXALTA® and HEMOFIL® are trademarks of Baxalta Incorporated, a wholly-owned, indirect subsidiary of Shire plc.
SHIRE and the Shire Logo are trademarks or registered trademarks of Shire Pharmaceutical Holdings Ireland Limited or its affiliates.
Baxalta US Inc.
Lexington, MA 024221 USA
U.S. License No. 2020Revised: Jun 2018
-
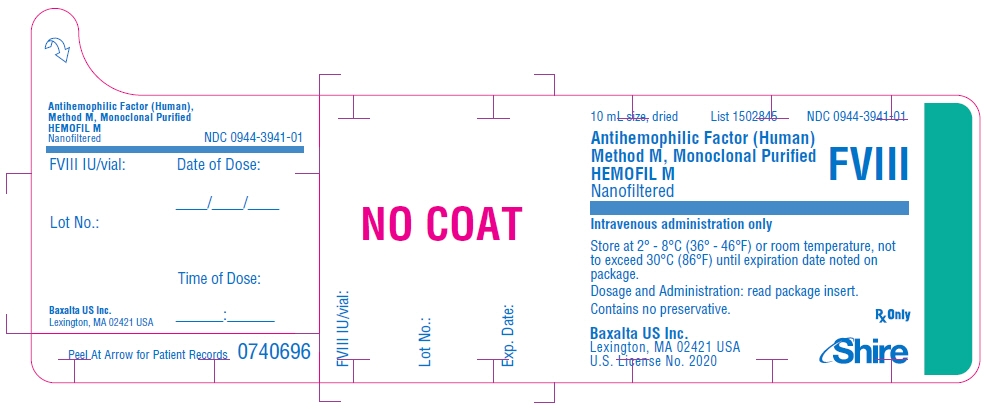
PRINCIPAL DISPLAY PANEL - 10 mL Bottle Label - 250 iU
10 mL size, dried
List 1502845
NDC: 0944-3941-01Antihemophilic Factor (Human)
Method M, Monoclonal Purified
HEMOFIL M
NanofilteredFVIII
Intravenous administration only
Store at 2° - 8°C (36° - 46°F) or room temperature, not
to exceed 30°C (86°F) until expiration date noted on
package.Dosage and Administration: read package insert.
Contains no preservative.Baxalta US Inc.
Lexington, MA 02421 USA
U.S. License No. 2020Rx Only
Shire
-
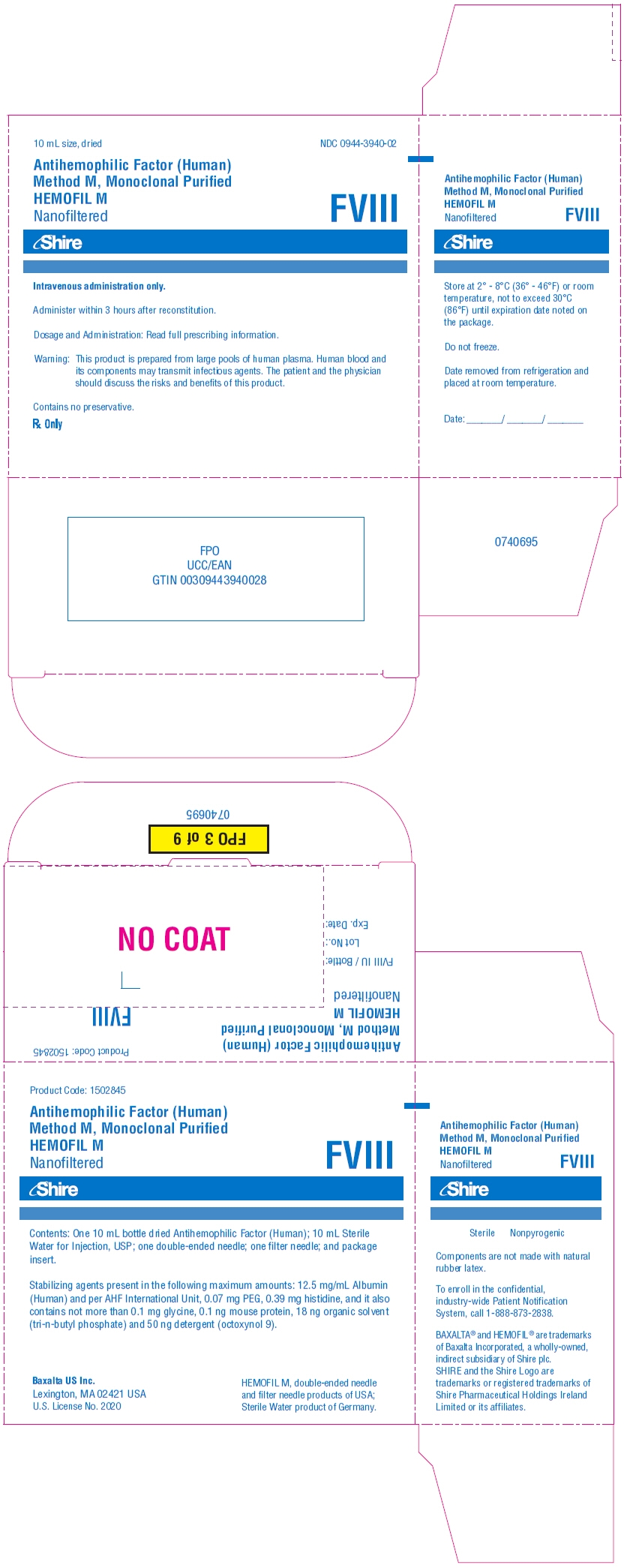
PRINCIPAL DISPLAY PANEL - Kit Carton - 250 iU
10 mL size, dried
NDC: 0944-3940-02
Antihemophilic Factor (Human)
Method M, Monoclonal Purified
HEMOFIL M
NanofilteredFVIII
Shire
Intravenous administration only.
Administer within 3 hours after reconstitution.
Dosage and Administration: Read full prescribing information.
Warning: This product is prepared from large pools of human plasma. Human blood and
its components may transmit infectious agents. The patient and the physician
should discuss the risks and benefits of this product.Contains no preservative.
Rx Only
-
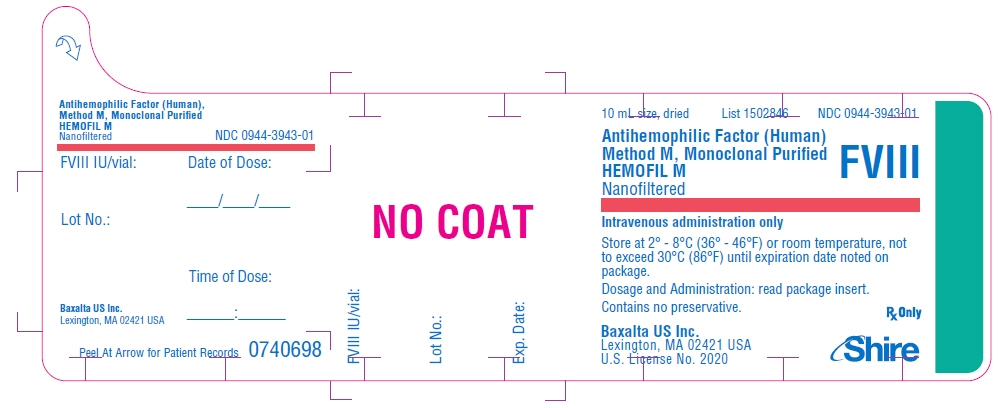
PRINCIPAL DISPLAY PANEL - 10 mL Bottle Label - 500 iU
10 mL size, dried
List 1502846
NDC: 0944-3943-01Antihemophilic Factor (Human)
Method M, Monoclonal Purified
HEMOFIL M
NanofilteredFVIII
Intravenous administration only
Store at 2° - 8°C (36° - 46°F) or room temperature, not
to exceed 30°C (86°F) until expiration date noted on
package.Dosage and Administration: read package insert.
Contains no preservative.Baxalta US Inc.
Lexington, MA 02421 USA
U.S. License No. 2020Rx Only
Shire
-
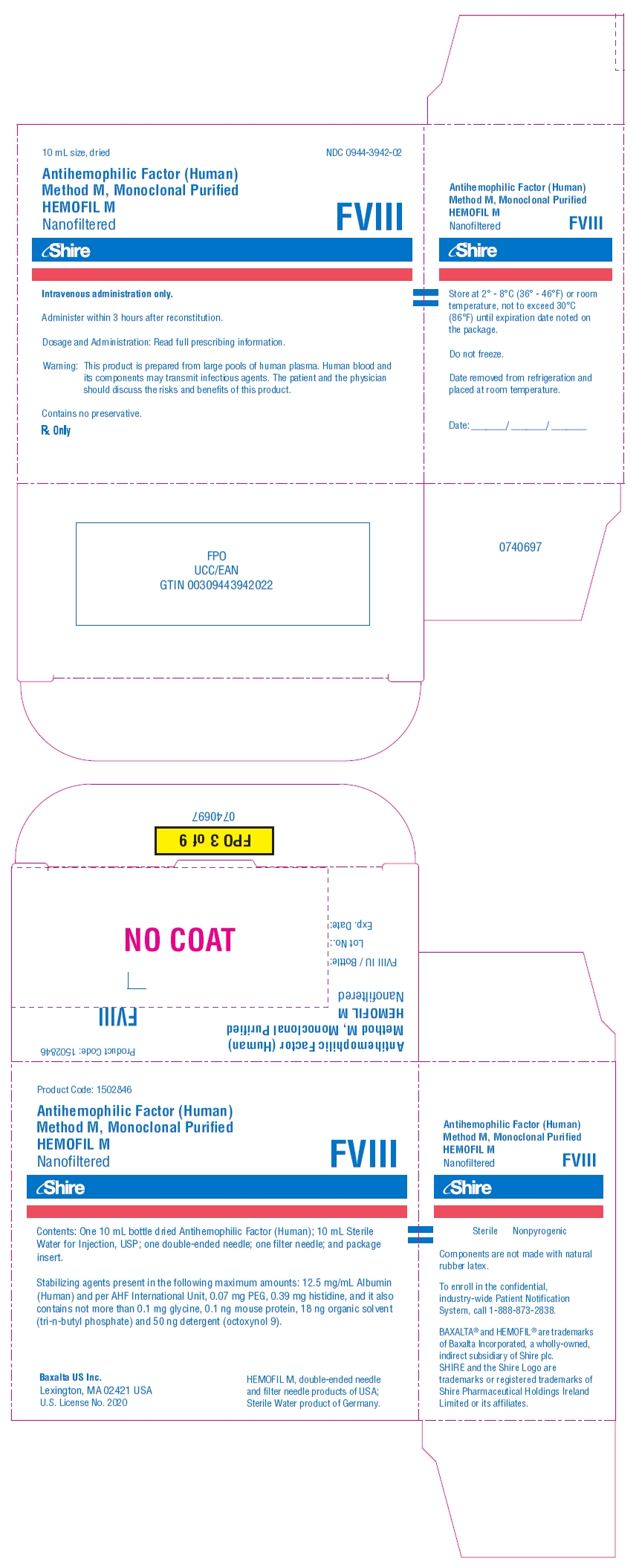
PRINCIPAL DISPLAY PANEL - Kit Carton - 500 iU
10 mL size, dried
NDC: 0944-3942-02
Antihemophilic Factor (Human)
Method M, Monoclonal Purified
HEMOFIL M
NanofilteredFVIII
Shire
Intravenous administration only.
Administer within 3 hours after reconstitution.
Dosage and Administration: Read full prescribing information.
Warning: This product is prepared from large pools of human plasma. Human blood and
its components may transmit infectious agents. The patient and the physician
should discuss the risks and benefits of this product.Contains no preservative.
Rx Only
-
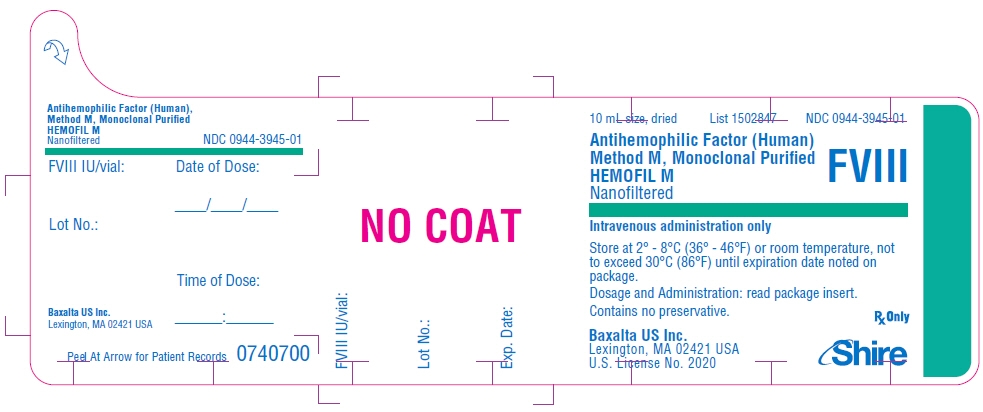
PRINCIPAL DISPLAY PANEL - 10 mL Bottle Label - 1000 iU
10 mL size, dried
List 1502847
NDC: 0944-3945-01Antihemophilic Factor (Human)
Method M, Monoclonal Purified
HEMOFIL M
NanofilteredFVIII
Intravenous administration only
Store at 2° - 8°C (36° - 46°F) or room temperature, not
to exceed 30°C (86°F) until expiration date noted on
package.Dosage and Administration: read package insert.
Contains no preservative.Baxalta US Inc.
Lexington, MA 02421 USA
U.S. License No. 2020Rx Only
Shire
-
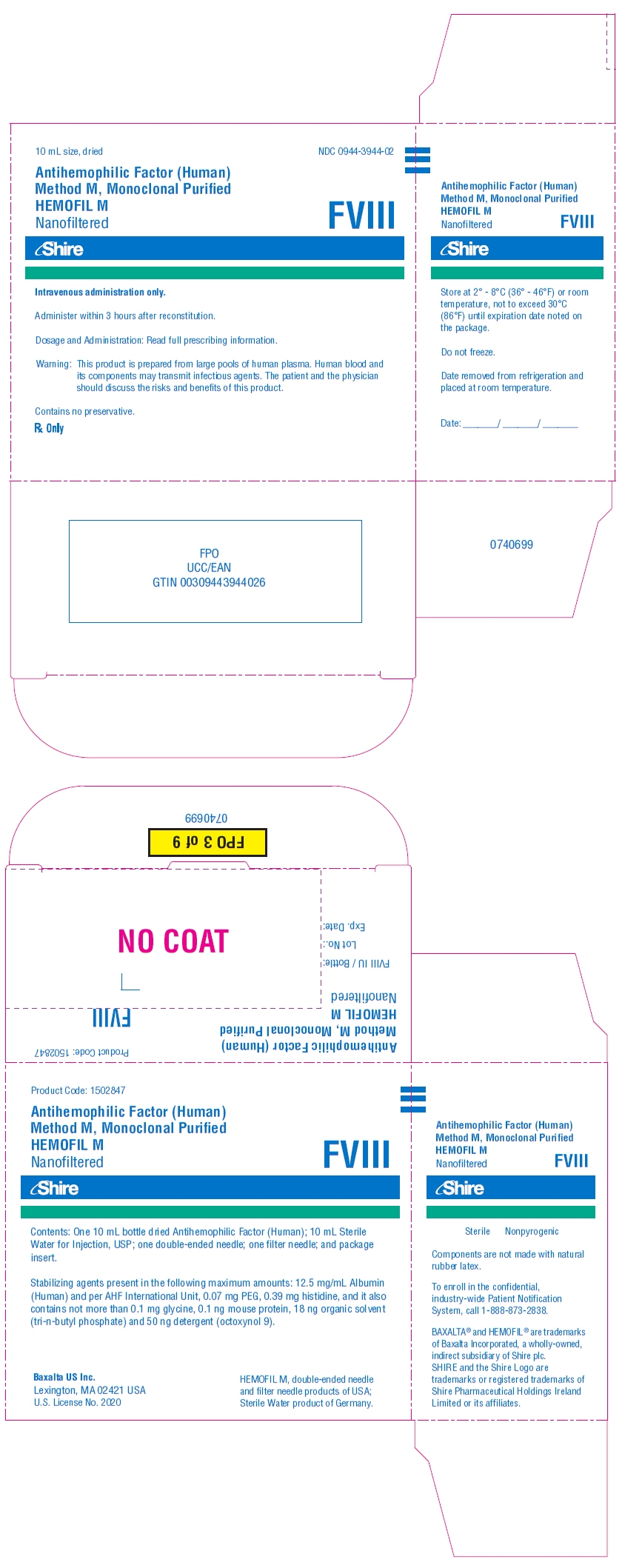
PRINCIPAL DISPLAY PANEL - Kit Carton - 1000 iU
10 mL size, dried
NDC: 0944-3944-02
Antihemophilic Factor (Human)
Method M, Monoclonal Purified
HEMOFIL M
NanofilteredFVIII
Shire
Intravenous administration only.
Administer within 3 hours after reconstitution.
Dosage and Administration: Read full prescribing information.
Warning: This product is prepared from large pools of human plasma. Human blood and
its components may transmit infectious agents. The patient and the physician
should discuss the risks and benefits of this product.Contains no preservative.
Rx Only
-
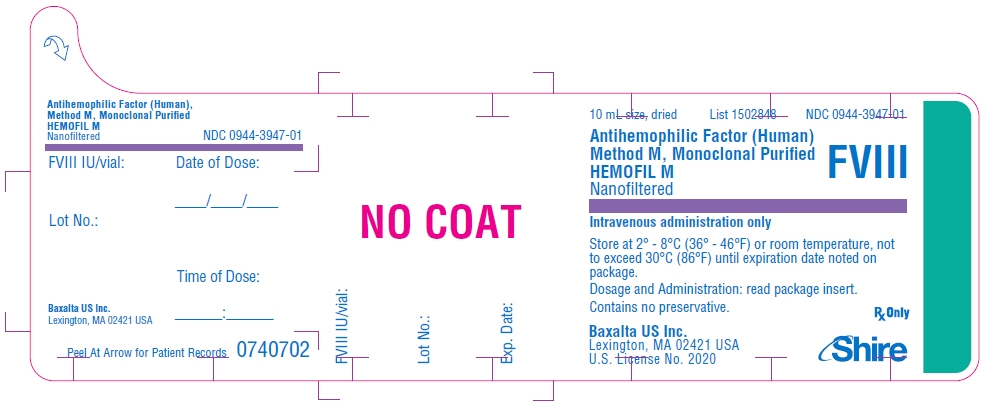
PRINCIPAL DISPLAY PANEL - 10 mL Bottle Label - 1700 iU
10 mL size, dried
List 1502848
NDC: 0944-3947-01Antihemophilic Factor (Human)
Method M, Monoclonal Purified
HEMOFIL M
NanofilteredFVIII
Intravenous administration only
Store at 2° - 8°C (36° - 46°F) or room temperature, not
to exceed 30°C (86°F) until expiration date noted on
package.Dosage and Administration: read package insert.
Contains no preservative.Baxalta US Inc.
Lexington, MA 02421 USA
U.S. License No. 2020Rx Only
Shire
-
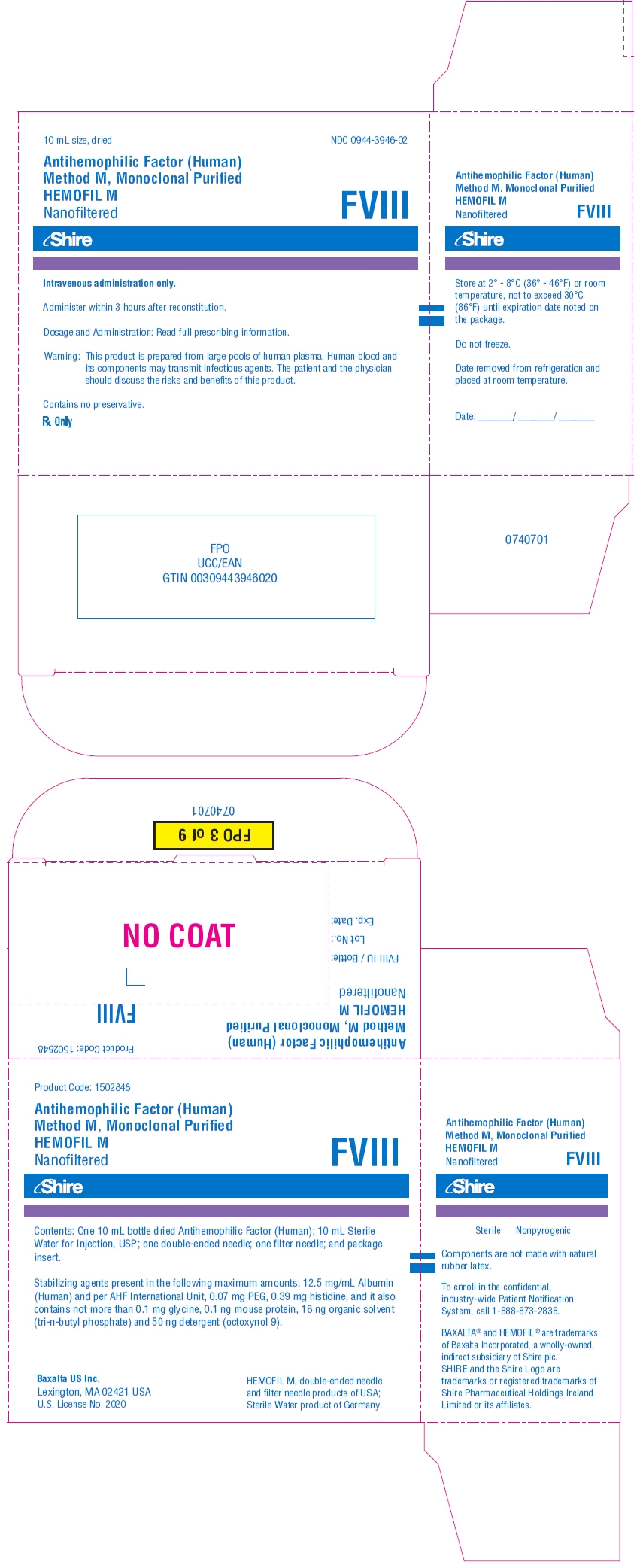
PRINCIPAL DISPLAY PANEL - Kit Carton - 1700 iU
10 mL size, dried
NDC: 0944-3946-02
Antihemophilic Factor (Human)
Method M, Monoclonal Purified
HEMOFIL M
NanofilteredFVIII
Shire
Intravenous administration only.
Administer within 3 hours after reconstitution.
Dosage and Administration: Read full prescribing information.
Warning: This product is prepared from large pools of human plasma. Human blood and
its components may transmit infectious agents. The patient and the physician
should discuss the risks and benefits of this product.Contains no preservative.
Rx Only
-
PRINCIPAL DISPLAY PANEL - 10 mL Vial Label
Sterile Water Vial Label Baxter 10 mL
NDC: 0338-0001-68
Nonpyrogenic
Single-Dose Container
10 mL
Sterile Water for Injection, USP
for reconstitution of accompanying product
Do not use unless clear. No antimicrobial agent or other
substance has been added. Do not use for intravascular
injection without making approximately isotonic by
addition of suitable solute. Discard unused portion.
Rx Only.
This Product Contains Dry Natural Rubber.
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Imported by
Baxter Corporation
Toronto, Ontario
-
INGREDIENTS AND APPEARANCE
HEMOFIL M
antihemophilic factor human kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-3940 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-3940-02 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 10 mL Part 2 1 BOTTLE 10 mL Part 1 of 2 HEMOFIL M
antihemophilic factor human powder, for solutionProduct Information Item Code (Source) NDC: 0944-3941 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR HUMAN (UNII: 839MOZ74GK) (ANTIHEMOPHILIC FACTOR HUMAN - UNII:839MOZ74GK) ANTIHEMOPHILIC FACTOR HUMAN 250 [iU] in 10 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) HISTIDINE (UNII: 4QD397987E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-3941-01 10 mL in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101448 02/23/1988 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC: 0338-0764 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 10 mL in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0764-61 10 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101448 02/23/1988 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101448 02/23/1988 HEMOFIL M
antihemophilic factor human kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-3942 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-3942-02 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 10 mL Part 2 1 BOTTLE 10 mL Part 1 of 2 HEMOFIL M
antihemophilic factor human powder, for solutionProduct Information Item Code (Source) NDC: 0944-3943 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR HUMAN (UNII: 839MOZ74GK) (ANTIHEMOPHILIC FACTOR HUMAN - UNII:839MOZ74GK) ANTIHEMOPHILIC FACTOR HUMAN 500 [iU] in 10 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) HISTIDINE (UNII: 4QD397987E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-3943-01 10 mL in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101448 02/23/1988 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC: 0338-0764 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 10 mL in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0764-61 10 mL in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101448 02/23/1988 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101448 02/23/1988 HEMOFIL M
antihemophilic factor human kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-3944 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-3944-02 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 10 mL Part 2 1 BOTTLE 10 mL Part 1 of 2 HEMOFIL M
antihemophilic factor human powder, for solutionProduct Information Item Code (Source) NDC: 0944-3945 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR HUMAN (UNII: 839MOZ74GK) (ANTIHEMOPHILIC FACTOR HUMAN - UNII:839MOZ74GK) ANTIHEMOPHILIC FACTOR HUMAN 1000 [iU] in 10 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) HISTIDINE (UNII: 4QD397987E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-3945-01 10 mL in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101448 02/23/1988 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC: 0338-0764 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 10 mL in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0764-61 10 mL in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101448 02/23/1988 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101448 02/23/1988 HEMOFIL M
antihemophilic factor human kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0944-3946 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-3946-02 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 10 mL Part 2 1 BOTTLE 10 mL Part 1 of 2 HEMOFIL M
antihemophilic factor human powder, for solutionProduct Information Item Code (Source) NDC: 0944-3947 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR HUMAN (UNII: 839MOZ74GK) (ANTIHEMOPHILIC FACTOR HUMAN - UNII:839MOZ74GK) ANTIHEMOPHILIC FACTOR HUMAN 1700 [iU] in 10 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) HISTIDINE (UNII: 4QD397987E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0944-3947-01 10 mL in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101448 02/23/1988 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC: 0338-0764 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 10 mL in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0764-61 10 mL in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101448 02/23/1988 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101448 02/23/1988 Labeler - BAXALTA US Inc. (079887619) Establishment Name Address ID/FEI Business Operations BAXALTA US Inc. 085206634 MANUFACTURE(0944-3940, 0944-3942, 0944-3944, 0944-3946) Establishment Name Address ID/FEI Business Operations BAXALTA US Inc. 009471603 MANUFACTURE(0944-3940, 0944-3942, 0944-3944, 0944-3946)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.