CHENODAL- chenodiol tablet, film coated
Chenodal by
Drug Labeling and Warnings
Chenodal by is a Prescription medication manufactured, distributed, or labeled by Manchester Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
PRINCIPAL DISPLAY PANEL

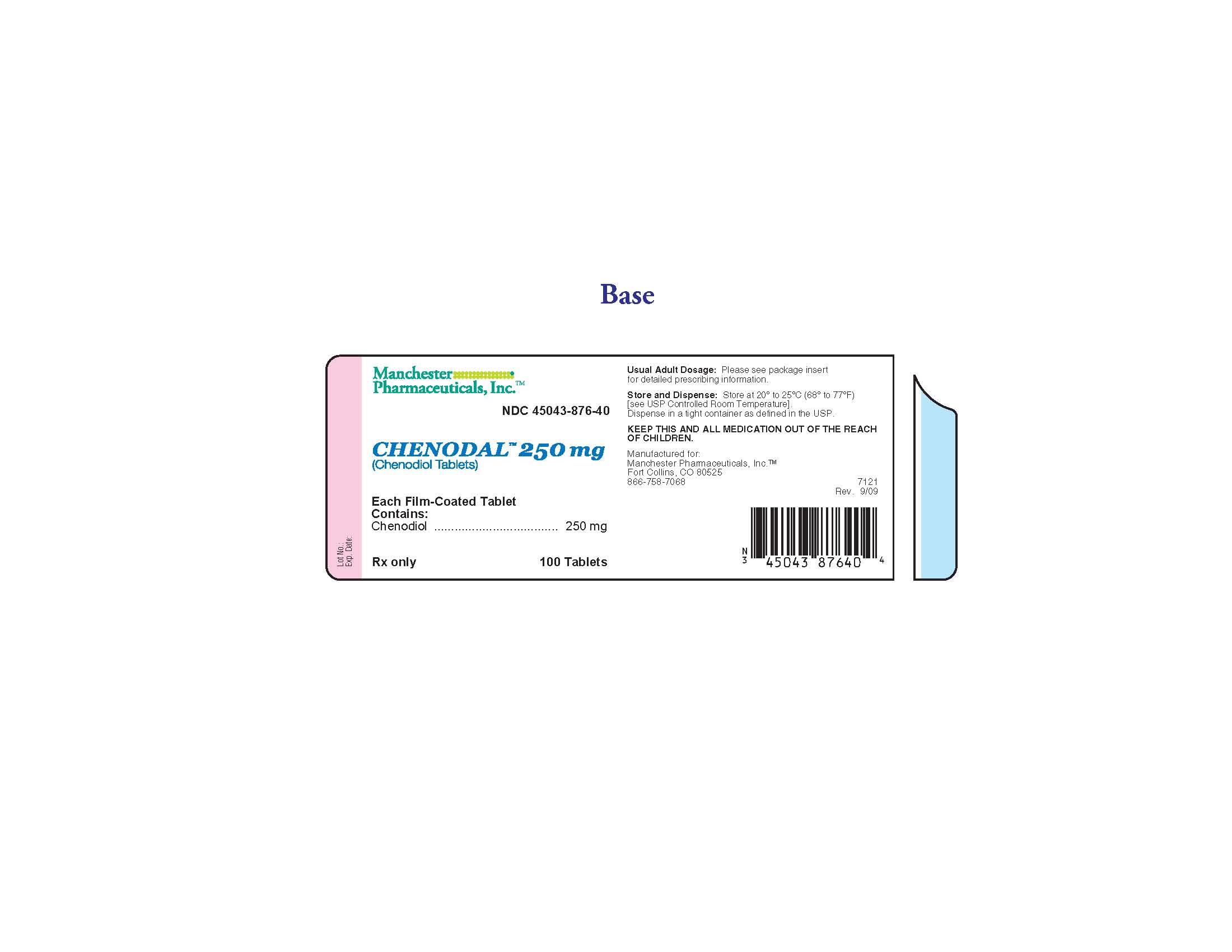
Rx only
NDC: 45043-876-40
Chenodal 250 mg
(Chenodiol Tablets)
100 Tablets
Manchester Pharmaceuticals, Inc.
Each Film-Coated Tablet Contains: Chenodiol 250 mg
Usual Adult Dosage: Please see package insert for detailed prescribing information.
Store and Dispense: Store at 200 to 250C (680 to 770F). [see USP Controlled Room Temperature]. Dispense in a tight container as defined in the USP.
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
Manufactured for: Manchester Pharmaceuticals, Inc.
Fort Collins, CO 80525
866-758-7068
Bar code
45043 87640
Lot No.:
Exp. Date:
-
SPECIAL NOTE
Because of the potential hepatoxicity of Chenodal, poor response rate in
some subgroups of Chenodal treated patients, and an increased rate of a need
for cholecystectomy in other Chenodal treated subgroups, Chenodal is not an
appropriate treatment for many patients with gallstones. Chenodal should be
reserved for carefully selected patients and treatment must be accompanied by
systematic monitoring for liver function alterations. Aspects of patient
selection, response rates and risks versus benefits are given in the insert.
-
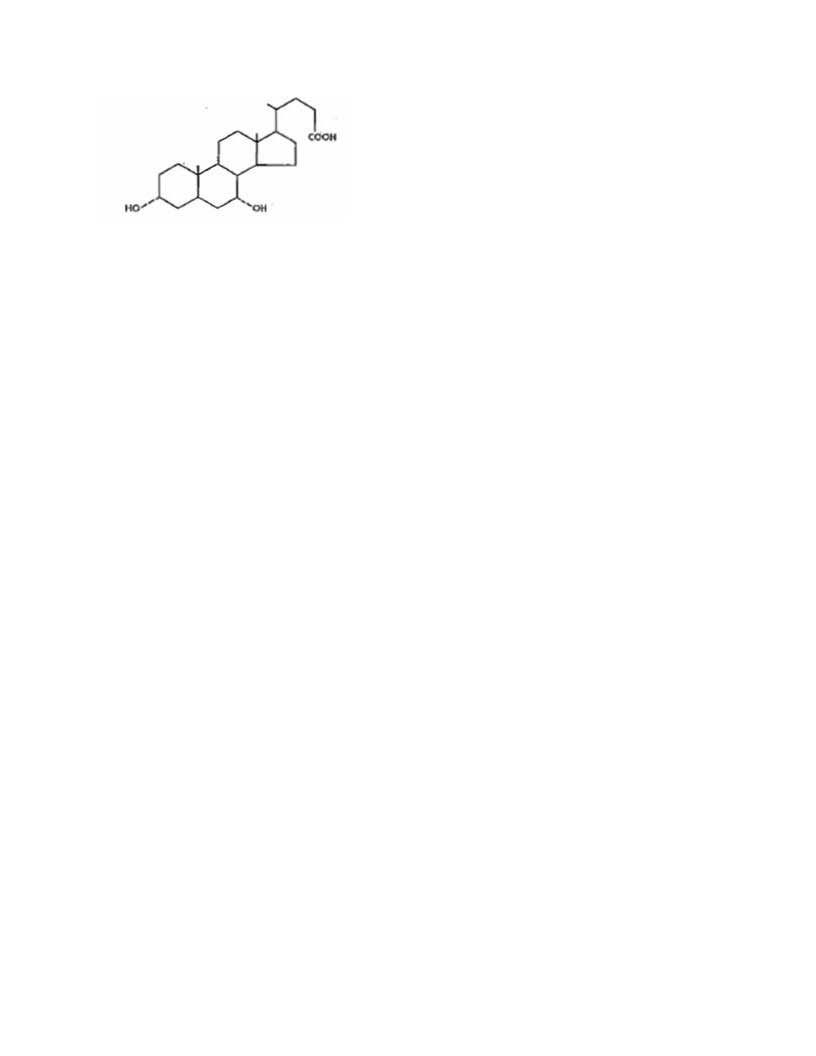
DESCRIPTION
Chenodiol is the non-proprietary name for chenodeoxycholic acid,
a naturally occurring human bile acid. It is a bitter-tasting white powder
consisting of crystalline and amorphous particles freely soluble in methanol,
acetone and acetic acid and practically insoluble in water. Its chemical name is
3α, 7α-dihydroxy-5β-cholan-24-oic acid (C24H40O4), it has a molecular weight of
392.58, and its structure is shown below;
Chenodiol film-coated tablets for oral administration contain 250 mg of
chenodiol.
Inactive ingredients: pregelatinized starch; silicon dioxide;
microcrystalline cellulose, sodium starch glycollate; and magnesium stearate;
the thin-film coating contains: opadry YS-2-7035 [consisting of methylcellulose
and glycerin] and sodium lauryl sulfate
-
CLINICAL PHARMACOLOGY
At therapeutic doses, chenodiol suppresses hepatic synthesis of
both cholesterol and cholic acid, gradually replacing the latter and its
metabolite, deoxycholic acid in an expanded bile acid pool. These actions
contribute to biliary cholesterol desaturation and gradual dissolution of
radiolucent cholesterol gallstones in the presence of a gall-bladder visualized
by oral cholecystography. Chenodiol has no effect on radiopaque (calcified)
gallstones or on radiolucent bile pigment stones.
Chenodiol is well absorbed from the small intestine and taken up by the liver
where it is converted to its taurine and glycine conjugates and secreted in
bile. Owing to 60 % to 80% first-pass hepatic clearance, the body pool of
chenodiol resides mainly in the enterohepatic circulation; serum and urinary
bile acid levels are not significantly affected during chenodiol therapy.
At steady-state, an amount of chenodiol near the daily dose escapes to the
colon and is converted by bacterial action to lithocholic acid. About 80% of the
lithocholate is excreted in the feces; the remainder is absorbed and converted
in the liver to its poorly absorbed sulfolithocholyl conjugates. During
chenodiol therapy there is only a minor increase in biliary lithocholate, while
fecal bile acids are increased three- to fourfold.
Chenodiol is unequivocally hepatotoxic in many animal species, including
sub-human primates at doses close to the human dose. Although the theoretical
cause is the metabolite, lithocholic acid, an established hepatotoxin, and man
has an efficient mechanism for sulfating and eliminating this substance, there
is some evidence that the demonstrated hepatotoxicity is partly due to chenodiol
per se. The hepatotoxicity of
lithocholic acid is characterized biochemically and morphologically as
cholestatic.
Man has the capacity to form sulfate conjugates of lithocholic acid.
Variation in this capacity among individuals has not been well established and a
recent published report suggests that patients who develop chenodiol-induced
serum aminotransferase elevations are poor sulfators of lithocholic acid (see ADVERSE
REACTIONS and WARNINGS).
General Clinical Results
Both the desaturation of bile and the clinical dissolution of cholesterol gallstones are dose-related. In
the National Cooperative Gallstone Study (NCGS) involving 305 patients in each
treatment group, placebo and chenodiol dosages of 375 mg and 750 mg per day were
associated with complete stone dissolution in 0.8%, 5.2% and 13.5%,
respectively, of enrolled subjects over 24 months of treatment. Uncontrolled
clinical trials using higher doses than those used in the NCGS have shown
complete dissolution rates of 28 to 38% of enrolled patients receiving body
weight doses of from 13 to 16 mg/kg/day for up to 24 months. In a prospective
trial using 15 mg/kg/day, 31% enrolled surgical-risk patients treated more than
six months (n = 86) achieved complete confirmed dissolutions.
Observed stone dissolution rates achieved with chenodiol treatment are higher
in subgroups having certain pretreatment characteristics. In the NCGS, patients
with small {less than 15 mm in diameter} radiolucent stones, the observed rate
of complete dissolution was approximately 20% on 750 mg/day. In the uncontrolled
trails using 13 to 16 mg/kg/day doses of chenodiol, the rates of complete
dissolution for small radiolucent stones ranged from 42% to 60%. Even higher
dissolution rates have been observed in patients with small floatable stones.
(See Floatable versus Nonfloatable Stones, below). Some
obese patients and occasional normal weight patients fail to achieve bile
desaturation even with doses of chenodiol up to 19 mg/kg/day for unknown
reasons. Although dissolution is generally higher with increased dosage of
chenodiol, doses that are too low are associated with increased cholecystectomy
rates (see ADVERSE REACTIONS).
Stones have recurred within five years in about 50% of patients following
complete confirmed dissolutions. Although retreatment with chenodiol has proven
successful in dissolving some newly formed stones, the indications for and
safety of retreatment are not well defined. Serum aminotransferase elevations
and diarrhea have been notable in all clinical trials and are dose-related
(refer to ADVERSE REACTIONS and WARNINGSsections
for full information).Floatable versus Nonfloatable Stones
A major finding in clinical trials was a difference between floatable and nonfloatable stones,
with respect to both natural history and response to chenodiol. Over the
two-year course of the National Cooperative Gallstone Study (NCGS), placebo –
treated patients with floatable stones (n = 47) had significantly higher rates
of biliary pain and cholecystectomy than patients with nonfloatable stones (n =
258) (47% versus 27% and 19%versus 4%, respectively). Chenodiol treatment (750
mg/day) compared to placebo was associated with a significant reduction in both
biliary pain and the cholecystectomy rates in the group with floatable stones
(27% versus 47% and 1.5% versus 19%, respectively). In an uncontrolled clinical
trial using 15 mg/kg/day, 70% of the patients with small (less than 15 mm)
floatable stones (n = 10) had complete confirmed dissolution.
In the NCGS in patients with nonfloatable stones, chenodiol produced no
reduction in biliary pain and showed a tendency to increase the cholecystectomy
rate (8% versus 4%). This finding was more pronounced with doses of chenodiol
below 10 mg/kg. The subgroup of patients with nonfloatable stones and a history
of biliary pain had the highest rates of cholecystectomy and aminotransferase
elevations during chenodiol treatment. Except for the NCGS subgroup with
pretreatment biliary pain, dose-related aminotransferase elevations and diarrhea
have occurred with equal frequency in patients with floatable or nonfloatable
stones. In the uncontrolled clinical trial mentioned above, 27% of the patients
with nonfloatable stones (n = 59) had complete confirmed dissolutions, including
35% with small (less than 15 mm)(n= 40) and only 11% with large, nonfloatable
stones (n= 19).
Of 916 patients enrolled NCGS, 17.6% had stones seen in upright form
(horizontal X-ray beam) to float in the dye-laden bile during oral
cholecystography using iopanoic acid. Other investigators report similar
findings. Floatable stones are not detected by ultrasonography in the absence
for dye. Chemical analysis has shown floatable stones to be essentially pure
cholesterol).
Other Radiographic and Laboratory Features
Radiolucent stones may have rims or centers of opacity representing
calcification. Pigment stones and partially calcified radiolucent stones do not
respond to chenodiol. Subtle calcification can sometimes be detected in flat
film X-rays, if not obvious in the oral cholecystogram. Among nonfloatable
stones, cholesterol stones are more apt than pigment stones to be smooth
surfaced, less than 0.5 cm in diameter, and to occur in numbers less than 10. As
stone size number and volume increase, the probability of dissolution within 24
months decreases. Hemolytic disorders, chronic alcoholism, biliary cirrhosis and
bacterial invasion of the biliary system predispose to pigment gallstone
formation. Pigment stones of primary biliary cirrhosis should be suspected in
patients with elevated alkaline phosphates, especially if positive
anti-mitochondrial antibodies are present. The presence of microscopic
cholesterol crystals in aspirated gallbladder bile, and demonstration of
cholesterol super saturation by bile lipid analysis increase the likelihood that
the stones are cholesterol stones.
- PATIENT SELECTION
-
Evaluation of Surgical Risk
Surgery offers the advantage of immediate and permanent stone removal, but carries a fairly high risk. In some patients, about 5% of cholecystectomized patients have residual symptoms or retained common duct stones. The spectrum to surgical risk varies as a function of age and the presence of disease other than cholelithiasis. Selected tabulation of results from the National Halothane Study (JAMA, 1968, 197:775-778) is shown below: the study included 27,600 cholecystectomies.
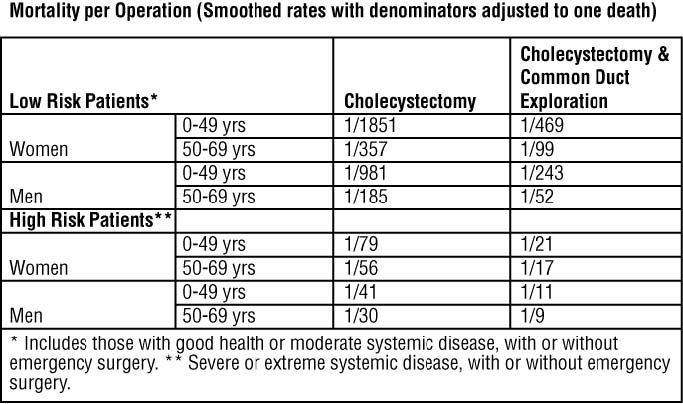
Women in good health, or having only moderate systemic disease, under 49 years of age have the lowest rate (0.054%); men in all categories have a surgical mortality rate twice that of women; common duct exploration quadruples the rates in all categories; the rates rise with each decade of life and increase tenfold or more in all categories with severe or extreme systemic disease.
Relatively young patients requiring treatment might be better treated by surgery than with chenodiol, because treatment with chenodiol, even if successful, is associated with a high rate of recurrence. The long-term consequences of repeated courses of chenodiol in terms of liver toxicity, neoplasia and elevated cholesterol levels are not known. Watchful waiting has the advantage that no therapy may ever be required. For patients with silent or minimally symptomatic stones, the rate of moderate to severe symptoms or gallstone complications is estimated to be between 2% and 6% per year, leading to a cumulative rate of 7% and 27% in five years. Presumably the rate is higher for patients already having symptoms. -
INDICATIONS AND USAGE
Chenodal (chenodiol tablets) is indicated for patients with radiolucent stones in well-opacifying gallbladders, in whom selective surgery would be undertaken except for the presence of increased surgical risk due to systemic disease or age. The likelihood of successful dissolution is far greater if the stones are floatable or small. For patients with nonfloatable stones, dissolution is less likely and added weight should be given to the risk that more emergent surgery might result form a delay due to unsuccessful treatment. Safety of use beyond 24 months is not established. Chenodiol will not dissolve calcified (radiopaque) or radiolucent bile pigment stones.
-
CONTRAINDICATIONS
Chenodal (chenodiol tablets) is contraindicated in the presence of know hepatocyte
dysfunction or bile ductal abnormalities such as intrahepatic cholestasis,
primary biliary cirrhosis or sclerosing cholangitits (see Warnings); a
gallbladder confirmed as nonvisualizing after two consecutive single doses of
dye; radiopaque stones; or gallstone complications or compelling reasons for
gallbladder surgery including unremitting acute cholecystitis, cholangitis,
biliary obstruction, gallstone pancreatitis, or biliary gastrointestinal
fistula.Pregnancy Category X
Chenodal (chenodiol tablets) may cause fetal harm when administered to a pregnant woman. Serious
hepatic, renal and adrenal lesions occurred in fetuses of female Rhesus monkeys
given 60 to 90 mg/kg/day (4 to 6 times the maximum recommended human dose, MRHD)
from day 21 to day 45 of pregnancy. Hepatic lesions also occurred in neonatal
baboons whose mothers had received 18 to 38 mg/kg ( 1 to 2 times the MRHD), all
during pregnancy. Fetal malformations were not observed. Neither fetal liver
damage nor fetal abnormalities occurred in reproduction studies in rats and
hamsters. No human data are available at this time. Chenodal (chenodiol tablets) is contraindicated
in women who are or may become pregnant. If this drug is used during pregnancy,
or if the patient becomes pregnant while taking this drug, the patient should be
apprised of the potential hazard to the fetus. -
WARNINGS
Safe use of chenodiol depends upon selection of patients without
pre-existing liver disease and upon faithful monitoring of serum
aminotransferase levels to detect drug-induced liver toxicity. Aminotransferase
elevations over three times the upper limit of normal have required
discontinuation of chenodiol in 2% to 3% of patients. Although clinical and
biopsy studies have not shown fulminant lesions, the possibility remains that an
occasional patient may develop serious hepatic disease. Three patients with
biochemical and histologic pictures of chronic active hepatitis while on
chenodiol, 375 mg/day or 750 mg/day, have been reported. The biochemical
abnormalities returned spontaneously to normal in two of the patients within 13
and 17 months; and after 17 months’ treatment with prednisone in the third.
Follow-up biopsies were not done; and the causal relationship of the drug could
not be determined. Another biopsied patient was terminated from therapy because
of elevated aminotransferase levels and a liver biopsy was interpreted as
showing active drug hepatitis.
One patient with sclerosing cholangitis, biliary cirrhosis and history of
jaundice died during chenodiol treatment for hepatic duct stones. Before
treatment, serum aminotransferase and alkaline phosphate levels were over twice
the upper limit of normal; within one month they rose to over 10 time normal.
Chenodiol was discontinued at seven weeks, when the patient was hospitalized
with advanced hepatic failure and E. coli peritonitis; death ensued at the eight
week. A contribution of chenodiol to the fatal outcome could not be ruled
out.
Epidemiologic studies suggest that bile acids might contribute to human colon
cancer, but direct evidence is lacking. Bile acids, including chenodiol and
lithocholic acid, have no carcinogenic potential in animal models, but have been
shown to increase the number of tumors when administered with certain know
carcinogens. The possibility that chenodiol therapy might contribute to colon
cancer in otherwise susceptible individuals cannot be ruled out. -
PRECAUTIONS
Information for patients
Patients should be counseled on the importance of periodic visits
for liver function tests and oral cholecystograms (or ultrasonograms) for
monitoring stone dissolution; they should be made aware of the symptoms of
gallstone complications and be warned to report immediately such symptoms to the
physician. Patients should be instructed on ways to facilitate faithful
compliance with the dosage regimen throughout the usual long term of therapy,
and on temporary doses reduction if episodes of diarrhea occur.
Drug interactions
Bile acid sequestering agents, such as cholestyramine and
colestipol, may interfere with the action of Chenodiol by reducing its
absorption. Aluminum-based antacids have been shown to absorb bile acids in
vitro and may be expected to interfere with Chenodiol in the same manner as the
sequestering agents. Estrogen, oral contraceptive and collaborate (and perhaps
other lipid-lowering drugs) increase biliary cholesterol secretion, and the
incidence of cholesterol gallstones hence may counteract the effectiveness of
Chenodiol.
Due to its hepatotoxicity, chenodiol can affect the pharmacodynamics of
coumarin and its derivatives, causing unexpected prolongation of the prothrombin
time and hemorrahages. Patients on concommitant therapy with chenodiol and
coumarin or its derivatives should be monitored carefully. If prolongation of
prothrombin time is observed, the coumarin dosage should be readjusted to give a
prothrombin time 1½ to 2 times normal. If necessary Chenodal (chenodiol tablets) should be
discontinued.Carcinogenesis, mutagenesis, impairment of fertility
A two-year oral study of chenodiol in rats failed to show a
carcinogenic potential at the tested levels of 15 to 60 mg/kg/day (1 to 4 times
the maximum recommended human dose, MRHD). It has been reported that chenodiol
given in long-term studies at oral doses up to 600 mg/kg/day (40 times the MRHD)
to rats and 1000 mg/kg/day (65 times the MRHD) to mice induced benign and
malignant liver cell tumors in female rats and cholangiomata in female rats and
male mice. Two-year studies of lithocholic acid ( a major metabolite of
chenodiol) in mice (125 to 250 mg/kg/day) and rats (250 and 500 mg/kg/day) found
it not to be carcinogenic. The dietary administration of Lithocholic acid to
chickens is reported to cause hepatic adenomatous hyperplasia.
-
ADVERSE REACTIONS
Hepatobiliary
Dose-related serum aminotransferase (mainly SGPT) elevations, usually not accompanied by rises in
alkaline phosphatase or bilirubin, occurred in 30% or more of patients treated with the recommended dose of Chenodiol. In most cases, these elevations were
minor (1 ½ to 3 times the upper limit of laboratory normal) and transient, returning to within the normal range within six months despite continued
administration of the drug. In 2% to 3% of patients, SGPT levels rose to over three times the upper limit of laboratory normal, recurred on rechallenge with
the drug, and required discontinuation of chenodiol treatment. Enzyme levels have returned to normal following withdrawal of chenodiol (see WARNINGS).
Morphologic studies of liver biopsies taken before and after 9 and 24 months of treatment with chenodiol have shown that 63% of the patients prior to
chenodiol treatment had evidence of intrahepatic cholestasis. Almost all pretreatment patients had electron microscopic abnormalities. By the ninth month
of treatment, reexamination of two-thirds of the patients showed an 89% incidence of the signs of intrahepatic cholestasis. Two of 89 patients at the
ninth month had lithocholate-like lesions in the canalicular membrane, although there were not clinical enzyme abnormalities in the face of continued treatment
and no change in Type 2 light microscopic parameters.
Increased Cholecystectomy Rate
NCGS patients with a history of biliary pain prior to treatment had higher cholecystectomy rates
during the study if assigned to low dosage chenodiol (375 mg/day) than if
assigned to either placebo or high dosage chenodiol (750 mg/day). The
association with low dosage chenodiol though not clearly a causal one, suggests
that patients unable to take higher doses of chenodiol may be at greater risk of
cholecystectomy.
Gastrointestinal
Dose-related diarrhea has been encountered in 30% to 40% of chenodiol-treated patients and may occur at any
time during treatment, but is most commonly encountered when treatment is initiated. Usually, the diarrhea is mild, translucent, well-tolerated and does
not interfere with therapy. Dose reduction has been required in 10% to 15% of patients, and in a controlled trial about half of these required a permanent
reduction in dose. Anti-diarrhea agents have proven useful in some patients.
Discontinuation of Chenodal (chenodiol tablets) because of failure to control diarrhea is to be expected in approximately 3% of patients treated. Steady epigastric pain with
nausea typical of lithiasis (biliary colic) usually is easily distinguishable from the crampy abdominal pain of drug-induced diarrhea.
Other less frequent, gastrointestinal side effects reported include urgency, cramps, heartburn, constipation, nausea, and vomiting, anorexic, epigastric
distress, dyspepsis, flatulence and nonspecific abdominal pain. - DRUG ABUSE AND DEPENDENCE
-
DOSAGE AND ADMINISTRATION
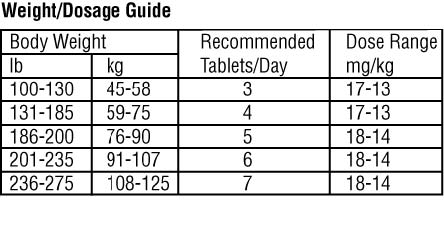
The recommended dose range for Chenodal (chenodiol tablets) is 13 to 16 mg/kg/day in two divided doses, morning and night, starting with 250 mg b.i.d. the first two
weeks and increasing by 250 mg/day each week thereafter until the recommended or maximum tolerated dose is reached. If diarrhea occurs during dosage buildup or
later in treatment, it usually can be controlled by temporary dosage adjustment until symptoms abate, after which the previous dosage usually is tolerated.
Dosage less than 10 mg/kg usually is ineffective and may be associated with increased risk of cholecystectomy, so is not recommended.
The optimal frequency of monitoring liver function tests is not known. It is suggested that serum aminotransferase levels should be monitored monthly for the
first three months and every three months thereafter during Chenodal (chenodiol tablets) administration. Under NCGS guidelines, if a minor, usually transient elevations
(1 ½ to3 three times the upper limit of normal) persisted longer than three to six months. Chenodiol was discontinued and resumed only after the
aminotransferase level returned to normal; however, allowing the elevations to persist over such an interval is not know to be safe. Elevations over three
times the upper limit of normal require immediate discontinuation of Chenodal (chenodiol tablets) and usually reoccur on challenge.
Serum cholesterol should be monitored at six months intervals. It may be advisable to discontinue Chenodal (chenodiol tablets) if cholesterol rises above the acceptable
age-adjusted limit for given patient.
Oral cholecystograms or ultrasonograms are recommend at six to nine month intervals to monitor response. Complete dissolutions should be confirmed by a
repeat test after one to three months continued Chenodal (chenodiol tablets) administration. Most patients who eventually achieve complete dissolution will show partial (or
complete) dissolution at the first on-treatment test. If partial dissolution is not seen by nine to 12 months, the likelihood of success of treating loner is
greatly reduced; Chenodal (chenodiol tablets) should be discontinued if there is no response by 18 months. Safety of use beyond 24 months is not established.
Stone recurrence can be expected within five years in 50% of cases. After confirmed dissolution, treatment generally should be stopped. Serial
cholecystograms or ultrasonograms are recommended to monitor for recurrence, keeping in mind that radiolucency and gallbladder function should be established
before starting another course of Chenodal (chenodiol tablets). A prophylactic doses is not established; reduced doses cannot be recommended; stones have recurred on 500
mg/day. Low cholesterol or carbohydrate diets, and dietary bran, have been reported to reduce biliary cholesterol; maintenance of reduced weight is
recommended to forestall stone recurrence.
-
HOW SUPPLIED
Chenodal™ 250 mg (chenodiol tablets) is available as white film-coated 250 mg tablets imprinted “MP” on one side and "250" on the other in bottles of 100,
NDC: 45043-876-40.
Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature].
Dispense in a tight container.
Manufactured for:
Manchester Pharmaceuticals, Inc.™
Fort Collins, CO 80525
866-758-7068 7121
Rev. 9/09 -
INGREDIENTS AND APPEARANCE
CHENODAL
chenodiol tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 45043-876(NDC:0722-7121) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Chenodiol (UNII: 0GEI24LG0J) (Chenodiol - UNII:0GEI24LG0J) Chenodiol 250 mg Product Characteristics Color white (White to Off-White) Score no score Shape ROUND Size 10mm Flavor Imprint Code MP;250 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 45043-876-40 100 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091019 10/01/2009 Labeler - Manchester Pharmaceuticals Inc. (832417641) Registrant - Manchester Pharmaceuticals Inc. (832417641) Establishment Name Address ID/FEI Business Operations Manchester Pharmaceuticals Inc. 832417641 relabel
Trademark Results [Chenodal]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CHENODAL 88936063 not registered Live/Pending |
Retrophin, Inc. 2020-05-27 |
 CHENODAL 77830559 4354250 Live/Registered |
RETROPHIN, INC. 2009-09-20 |
 CHENODAL 77652286 3755928 Live/Registered |
RETROPHIN, INC. 2009-01-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.