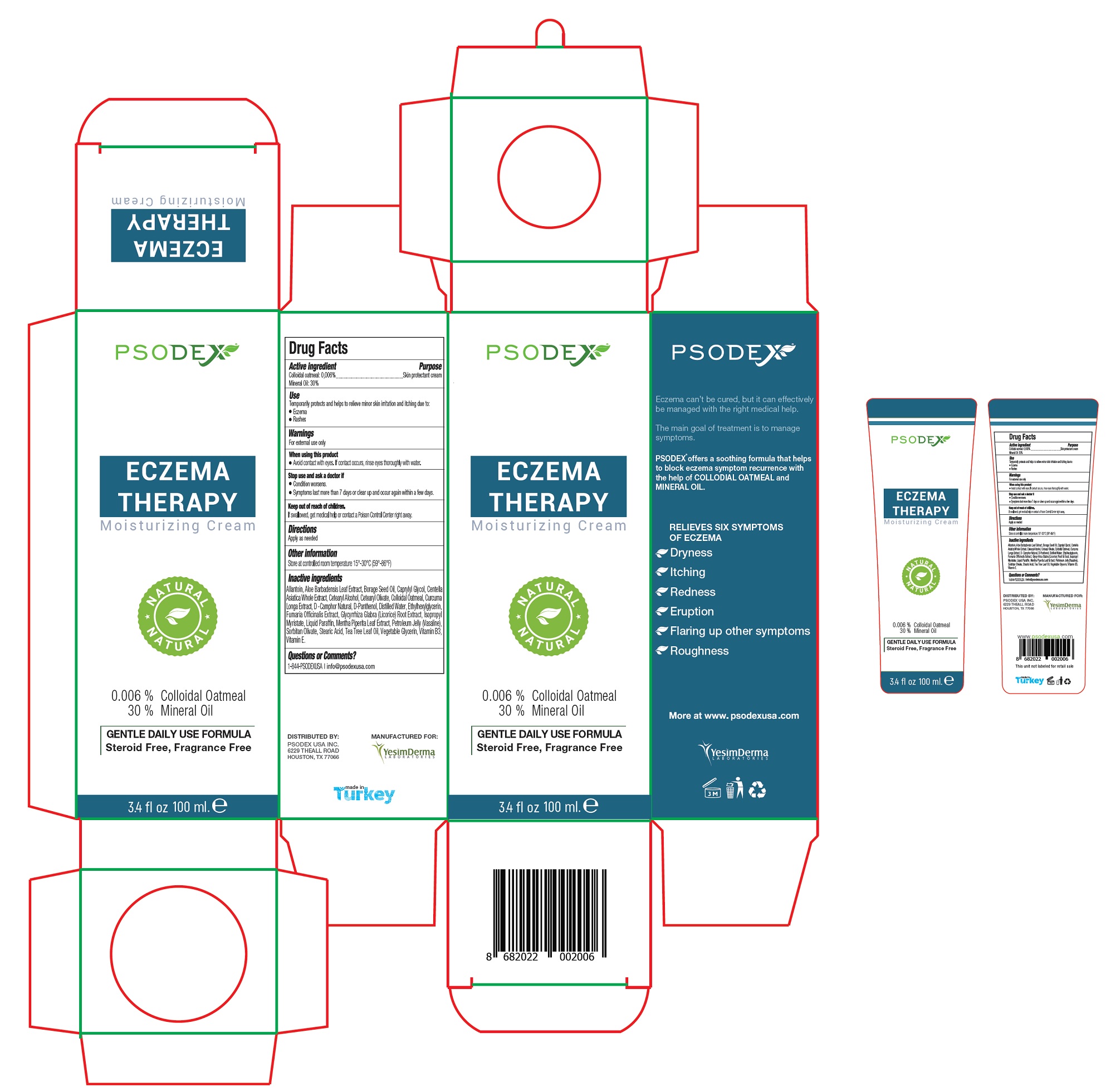
Psodex Eczema Therapy Moisturizing Cream
Psodex Eczema Therapy Moisturizing by
Drug Labeling and Warnings
Psodex Eczema Therapy Moisturizing by is a Otc medication manufactured, distributed, or labeled by Psodex USA Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PSODEX ECZEMA THERAPY MOISTURIZING- oatmeal, mineral cream
Psodex USA Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Psodex Eczema Therapy Moisturizing Cream
Use
Temporarily protects and helps to relieve minor skin irritation and itching due to:
- Eczema
- Rashes
Warnings
For external use only
When using this product
- Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Inactive ingredients
Allantoin, Aloe Barbadensis Leaf Extract, Borage Seed Oil, Caprylyl Glycol, Centella Asiatica Whole Extract, Cetearyl Alcohol, Cetearyl Olivate, Colloidal Oatmeal, Curcuma Longa Extract, D -Camphor Natural, D-Panthenol, Distilled Water, Ethylhexylglycerin, Fumaria Officinalis Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Isopropyl Myristate, Liquid Paraffin, Mentha Piperita Leaf Extract, Petroleum Jelly (Vasaline), Sorbitan Olivate, Stearic Acid, Tea Tree Leaf Oil, Vegetable Glycerin, Vitamin B3, Vitamin E
| PSODEX ECZEMA THERAPY MOISTURIZING
oatmeal, mineral cream |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Psodex USA Inc. (076051073) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.