CLEVOR- ropinirole hydrochloride solution
CLEVOR by
Drug Labeling and Warnings
CLEVOR by is a Animal medication manufactured, distributed, or labeled by Vetoquinol USA, Inc., Orion Corporation, Orion Pharma, Neuland Laboratories Limited, Holopack Verpackungstechnik GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- CAUTION:
-
DESCRIPTION:
CLEVOR (ropinirole ophthalmic solution) is a full dopamine agonist with selectivity for the dopamine D2-like receptor family. Each mL of CLEVOR contains 30 mg ropinirole (equivalent to 34.2 mg ropinirole hydrochloride). The chemical name of ropinirole hydrochloride is 2H-Indol-2- one, 4-[2- (dipropylamino)ethyl]-1,3-dihydro-, monohydrochloride. It is pale cream to yellow powder having a molecular weight of 296.84. The molecular formula is C16 H24 N2O ∙HCL and the structural formula is:
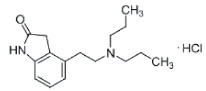
Inactive ingredients: citric acid monohydrate, sodium citrate, sodium chloride.
- INDICATION:
-
DOSAGE AND ADMINISTRATION:
This product should be administered by veterinary personnel.
Dosing Instructions:
Administer the appropriate number of eye drops topically according to Table 1. The number of eye drops administered corresponding to body weight results in a target dose of 3.75 mg/m2 (dose band 2.7 - 5.4 mg/m2). If the dog does not vomit within 20 minutes of the first dose, then a second dose may be administered.
Table 1. Dose Administration Body weight in kilograms Body weight in pounds Total number of eye drops Example administration 1.8 - 5 4 - 11.1 1 1 drop into either left or right eye 5.1 - 10 11.2 - 22.1 2 1 drop each eye 10.1 - 20 22.2 - 44.1 3 2 drops in one eye and 1 drop in the other eye 20.1 - 35 44.2 - 77.2 4 2 drops in each eye 35.1 - 60 77.3 - 132.3 6 An initial dose of 2 drops in each eye, followed 2 minutes later by 1 drop in each eye 60.1 - 100 132.4 - 220.5 8 An initial dose of 2 drops in each eye, followed 2 minutes later by 2 drops in each eye Dose Administration:
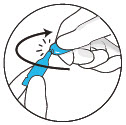
- Wear gloves and protective eye wear when handling or administering this product to prevent accidental exposure.
- Open the dropper by twisting off the tail.
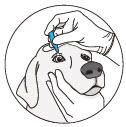
- Keep the dog's head steady in a slightly upright position.
- Hold the dropper in an upright position without touching the eye.
- Rest your finger on the forehead of your dog to maintain the distance between the dropper and the eye.
- Squeeze the prescribed number of drops in to the eye(s).
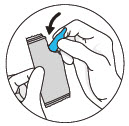
- CLEVOR is a single use dropper and is light sensitive.
- After administration, with gloves on, return the dropper to the aluminum pouch and place in the carton.

- If the dog does not vomit, a second dose can be given 20 minutes after administration of the first dose.
- This second dose is the same number of drops as the first dose.
- Thirty minutes after opening, with gloves on, dispose of dropper, aluminum pouch, and carton.
Refer to the Animal Safety Warnings section for treatment of protracted vomiting.
-
CONTRAINDICATIONS:
Do not use in dogs with central nervous system depression or seizures.
Do not use in cases of ingestion of sharp foreign objects, corrosive agents (acids or alkalis), volatile substances or organic solvents.
Do not use in cases with corneal ulceration, ocular irritation, or ocular injury.
Do not use when there is a known sensitivity to ropinirole or the inactive ingredients.
-
WARNINGS:
Human Safety Warnings:
Not for use in humans. Keep out of reach of children.
Wear gloves and protective eye wear when handling or administering this product to prevent accidental exposure. In case of accidental eye, oral or skin exposure, flush with water. If wearing contact lenses, eyes should be rinsed first, then remove contact lenses and continue rinsing. Remove contaminated clothing. Ropinirole is a dopamine agonist. Seek medical attention if accidental exposure occurs and show the package insert or label to the physician. Exposure to this drug may cause adverse reactions such as headache, nausea, vomiting, dizziness, orthostatic hypotension, and sleepiness. Avoid contact with the product if pregnant, planning to become pregnant, or breast-feeding, as exposure has been shown to have adverse effects on embryo-fetal development based on rodent studies.
Animal Safety Warnings:
This product should be administered by veterinary personnel.
Dogs should be monitored for CLEVOR-associated clinical signs, including protracted vomiting, salivation, muscle tremors, evidence of abdominal discomfort, lethargy, transient tachycardia, transient decrease in blood pressure and signs of ocular irritation, including conjunctival hyperemia, mild blepharospasm, and protrusion of the third eyelid. These clinical signs are related to the pharmacological action of ropinirole.
To stop protracted vomiting, administer metoclopramide (dopamine D2 antagonist) at a dose of 0.5 mg/kg intravenously (IV) or subcutaneously (SQ). Metoclopramide also decreases the prevalence of most CLEVOR-associated clinical signs.
-
PRECAUTIONS:
The safe use of CLEVOR has not been evaluated in dogs with cardiac disease or cardiovascular compromise. CLEVOR can cause transient tachycardia and transient decreased systolic blood pressure.
The safe use of CLEVOR has not been evaluated in dogs with hepatic impairment. CLEVOR is metabolized by the liver.
The safe use of CLEVOR has not been evaluated in dogs younger than 4.5 months of age and weight less than 4 pounds.
The safe use of CLEVOR has not been evaluated in dogs that are pregnant, lactating, or intended for breeding.
-
ADVERSE REACTIONS:
Safety was evaluated during a field study that enrolled 132 dogs (100 in the CLEVOR group and 32 in the vehicle control group). CLEVOR was administered as drops into the eyes at the dose as directed by the dosing table (see DOSAGE AND ADMINISTRATION). The following table shows the number of dogs exhibiting ocular, systemic, and clinical pathology adverse reactions.
Table 2: Adverse Reactions Reported During the Study (all dogs) Organ System Adverse Reaction CLEVOR
(N=100)Vehicle control
(N=32)- * Assessment performed 30 minutes after dose administration
- † Tachycardia resolved for most dogs within 4 hours after dose administration
- ‡ Urinalysis results were reported for only 86 dogs (66 administered CLEVOR and 20 control)
- § All three dogs had elevated alanine aminotransferase. Additionally, one of the dogs also had an elevated aspartate aminotransferase and another of the dogs also had an elevated alkaline phosphatase and total bilirubin.
Ocular Conjunctival hyperemia* 51 (51%) 6 (19%) Protrusion of the third eyelid* 38 (38%) 1 (3%) Conjunctival discharge* 30 (30%) 1 (3%) Blepharospasm* 19 (19%) 0 Conjunctival swelling* 3 (3%) 0 Scratching/rubbing of eyes* 4 (4%) 0 Corneal ulceration 1 (1%) 0 Corneal fluorescein uptake without corneal ulceration 1 (1%) 0 Systemic Lethargy 41 (41%) 0 Tachycardia (>160 beats per minute)*,† 14 (14%) 0 Vomiting duration longer than one hour 8 (8%) 0 Salivation 3 (3%) 1 (3%) Trembling 3 (3%) 0 Diarrhea or soft stool 2 (2%) 1 (3%) Anxious 1 (1%) 0 Borborygmi 1 (1%) 0 Clinical Pathology Crystalluria‡ 13 (20%) 3 (15%) Pyuriac‡ 12 (18%) 3 (15%) Increased liver enzymes§ 3 (3%) 0 Decreased blood glucose 2 (2%) 0 Increased prothrombin time 1 (1%) 0 Note: If any animal experienced the event more than once, only the first occurrence was tabulated.
To report suspected adverse events call 1(800) 835-9496, for technical assistance or to obtain a copy of the SDS, contact Vetoquinol USA, Inc. at 1 (800) 267-5707 or www.vetoquinolusa.com.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
-
CLINICAL PHARMACOLOGY:
Ropinirole is a full dopamine agonist with selectivity for the dopamine D2-like receptor family. Ropinirole induces vomiting by activating the D2-like receptors in the chemoreceptor trigger zone, located in the area postrema, which transmits the information to the vomiting center to trigger vomiting.
Absorption: Ropinirole solution is rapidly absorbed after ocular administration in dogs. The systemic bioavailability of the drug by this route of administration is 18 to 28%. The maximum concentration (Cmax) after ocular administration at the target dose level of 3.75 mg/m2 was 25.6 ± 7.75 ng/mL at 10 to 20 minutes (Tmax). Cmax in dogs that had to be re-dosed 20 minutes after the first administration was 34.2 ± 11.0 ng/mL at a Tmax of 10 to 60 minutes. Vomiting generally starts before the Cmax in plasma is reached. No direct correlation between the drug concentration in plasma and the duration of vomiting was observed after ocular administration.
Distribution: Ropinirole has a relatively large apparent volume of distribution. In dogs the volume of distribution (Vd) was 5.63 ± 1.40 L/kg after intravenous administration. The fraction bound to plasma proteins in dogs has been reported as 37%.1
Metabolism and Elimination: Ropinirole is mainly eliminated by hepatic metabolism.1,2
The half-life of elimination (t½) is approximately 4 hours after intravenous administration to dogs. Biotransformation occurs by dealkylation, hydroxylation and subsequent conjugation with glucuronic acid or oxidation to carboxylic acid.3,4 Excretion occurs mainly as metabolites in the urine.
-
EFFECTIVENESS:
Effectiveness was demonstrated in a multi-center, vehicle-controlled, randomized and masked field study in client-owned dogs of various breeds. Dogs enrolled in the study were 7 months to 15 years of age and weighed 1.9 to 66.3 kg. In this study, 132 dogs were enrolled and randomized to treatment with CLEVOR (n=100) or vehicle control (n=32). The effectiveness evaluation population included 99 CLEVOR treated dogs and 29 vehicle control dogs.
Dogs received a dose administered as drops into the eye(s) approximately 1 hour after a meal. The dose was administered according to weight (see Dosage and Administration). If the dog did not vomit within 20 minutes of the first dose, a second dose was administered.
Treatment success was defined for each dog as vomiting within 30 minutes of treatment. The percent of treatment success for CLEVOR was greater than vehicle control. The confidence interval around the estimated success rate 0.969 was (0.831, 0.995).
Table 3. Number and % Effectiveness for CLEVOR and Vehicle Control Time to Vomit CLEVOR ®
(n=99)Vehicle Control
(n=29)0-30 minutes 94 (95%) 0 (0%) The time to first vomition, the number of times the dog vomited, and the description of vomit were evaluated as secondary variables and support the effectiveness of CLEVOR for induction of vomiting in dogs. Eighty-five dogs (86%) in the CLEVOR group vomited within 20 minutes and therefore did not require a second dose. The time to first vomition ranged from 3 minutes to 37 minutes with a mean of 12 minutes. The duration between first and last vomiting episodes ranged from 0 to 108 minutes with a mean duration of 23 minutes. Eight dogs had vomiting episodes over a duration longer than an hour. Five of these eight dogs were administered an antiemetic (metoclopramide). The number of vomits ranged from 0 to 13 vomits with a mean of 4.8 vomits per dog. Of the dogs that vomited, 96% of the dogs produced a vomit containing the meal that had been fed approximately one hour prior to CLEVOR administration.
-
ANIMAL SAFETY:
In a laboratory study, 32 healthy Beagle dogs aged 4.5 - 5.5 months were administered CLEVOR at 1X, 3X, and 5X the proposed labeled dose of 2.7-5.4 mg/m2 (0.23-0.31 mg/kg), repeated once after 20 minutes [total dose of 8.5-10.6 mg/m2 (0.45-0.62 mg/kg)] (dose divided between both eyes), for 3 days.
Dogs in the CLEVOR groups generally vomited within 5 - 10 minutes of dose administration and none of the dogs in the saline group vomited. Vomiting occurred as often as every 1 - 2 minutes and lasted 1 - 2 hours. Additional systemic effects of CLEVOR included retching, salivation, hunched posture, tremors, labored breathing, lethargy, ventral and lateral recumbency, transient tachycardia, transient decrease in indirect systolic blood pressure (but not less than 125 mmHg), and a dose dependent decrease in body temperature (although all temperatures remained within normal limits). Local ocular effects of CLEVOR included ocular discharge, hyperemia, conjunctival erythema, blepharospasm, ptosis, third eyelid elevation, and positive corneal fluorescein staining. All CLEVOR-related observations resolved within 6 hours post-dosing. There was a dose dependent decrease in food consumption for dogs in the CLEVOR groups. Some dogs in the CLEVOR groups also had slight decreases in body weight. There were no effects on gross or microscopic pathology.
-
STORAGE CONDITIONS:
Store in the original package in order to protect from light at controlled room temperature 20-25˚C (68-77˚F) with excursions permitted to 15-30˚C (59-86˚F). After the first dosing, the opened dropper should be kept in the aluminum pouch. The content should be used and discarded within 30 minutes after opening the aluminum pouch.
-
HOW SUPPLIED:
CLEVOR is packaged in a unit-dose low density polyethylene dropper. Each dropper is packaged in an individual aluminum foil laminate pouch. The pouch/pouches are further packaged in a carton with the same number of leaflets as the number of unit-dose droppers.
Package sizes: Single package of 1 unit-dose dropper and multipackage of 5 unit-dose droppers.

-
REFERENCES:
1. Swagzdis J, et. al., (1986) Pharmacokinetics of dopamine-2 agonists in rats and dogs.
J Pharm Sci 75 (10): 925-928.2. Ramji J et al., (1999) Disposition of ropinirole in animals and man. Xenobiotica. 29 (3): 311-325.
3. Mico B et al., (1986) Functional group metabolism of dopamine-2 agonists: Conversion of 4-(2-Di-n-propylaminoethyl)-2-(3H)-indolone to 4-(2-Di-n-propylaminoethyl)-7-hydroxy-2-(3H)-indolone. J Pharm Sci 75 (10): 929-933.
4. Reavill C et al., (2000) Comparative pharmacological study of ropinirole (SKF-101468) and its metabolites in rats.
J Pharm Pharmacol 52 (9): 1129-1135. - SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 5 x 0.3 mL Bottle Box
Clevor®
(ropinirole
ophthalmic solution)30 mg/mL
For induction of vomiting in dogs only.
Caution: Federal law restricts this drug to
use by or on the order of a licensed veterinarian.Net contents: 5 x 0.3 mL; 5 droppers,
each dropper containing 9 mg ropiniroleApproved by FDA under NADA # 141-534
ORION
PHARMAvetoquinol

-
INGREDIENTS AND APPEARANCE
CLEVOR
ropinirole hydrochloride solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 17030-030 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ROPINIROLE HYDROCHLORIDE (UNII: D7ZD41RZI9) (ROPINIROLE - UNII:030PYR8953) ROPINIROLE 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17030-030-05 5 in 1 BOX, UNIT-DOSE 1 0.3 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141534 12/07/2020 Labeler - Vetoquinol USA, Inc. (106824209) Registrant - Orion Corporation, Orion Pharma (539763727) Establishment Name Address ID/FEI Business Operations Neuland Laboratories Limited 675596335 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Holopack Verpackungstechnik GmbH 343390324 MANUFACTURE, LABEL, PACK
Trademark Results [CLEVOR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CLEVOR 86968750 5140330 Live/Registered |
Orion Corporation 2016-04-08 |
 CLEVOR 85940840 not registered Dead/Abandoned |
Bayer Intellectual Property GmbH 2013-05-23 |
 CLEVOR 79001721 2955181 Dead/Cancelled |
Orion Corporation 2004-03-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.