ZOLMITRIPTAN tablet, orally disintegrating
Zolmitriptan by
Drug Labeling and Warnings
Zolmitriptan by is a Prescription medication manufactured, distributed, or labeled by Alembic Pharmaceuticals Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ZOLMITRIPTAN ORALLY DISINTEGRATING TABLETS safely and effectively. See full prescribing information for ZOLMITRIPTAN ORALLY DISINTEGRATING TABLETS.
ZOLMITRIPTAN orally disintegrating tablets
Initial U.S. Approval: 1997RECENT MAJOR CHANGES
Dosage and Administration (2.1, 2.3, 2.4) 09/2012
Warnings and Precautions (5.6) 09/2012
INDICATIONS AND USAGE
Zolmitriptan is a serotonin (5-HT)1B/1D receptor agonist (triptan) indicated for the acute treatment of migraine with or without aura in adults (1) (1)
Limitations of Use: (1)
- Use only after a clear diagnosis of migraine has been established (1)
- Not indicated for the prophylactic therapy of migraine (1)
- Not indicated for the treatment of cluster headache (1)
DOSAGE AND ADMINISTRATION
- Recommended starting dose: 1.25 mg or 2.5 mg (2.1)
- Maximum single dose: 5 mg (2.1)
- May repeat dose after 2 hours if needed; not to exceed 10 mg in any 24 hour period (2.1)
- Do not break zolmitriptan orally disintegrating tablets (2.2)
- Moderate or Severe Hepatic Impairment: 1.25 mg recommended (2.3, 8.6)
DOSAGE FORMS AND STRENGTHS
- Orally Disintegrating Tablets: 2.5 mg and 5 mg (3)
CONTRAINDICATIONS
- History of coronary artery disease (CAD)or coronary vasospasm (4)
- Symptomatic Wolff-Parkinson-White syndrome or other cardiac accessory conduction pathway disorders (4)
- History of stroke, transient ischemic attack, or hemiplegic or basilar migraine (4)
- Peripheral vascular disease (4)
- Ischemic bowel disease (4)
- Uncontrolled hypertension (4)
- Recent (within 24 hours) use of another 5-HT1 agonist (e.g., another triptan), or an ergotamine-containing medication (4)
- Monamine oxidase (MAO)-A inhibitor used in past 2 weeks (4)
- Known hypersensitivity to zolmitriptan (4)
WARNINGS AND PRECAUTIONS
- Myocardial Ischemia/Infarction, and Prinzmetal Angina: Perform cardiac evaluation in patients with multiple cardiovascular risk factors (5.1)
- Arrhythmias: Discontinue zolmitriptan if occurs (5.2)
- Chest/Throat/Neck/Jaw Pain, Tightness, and Pressure: Generally not associated with myocardial ischemia; evaluate for CAD in patients at high risk (5.3)
- Cerebral Hemorrhage, Subarachnoid Hemorrhage, and Stroke: Discontinue zolmitriptan if occurs (5.4)
- Gastrointestinal Ischemic Reactions and Peripheral Vasospastic Reactions: Discontinue zolmitriptan if occurs (5.5)
- Medication Overuse Headache: Detoxification may be necessary (5.6)
- Serotonin Syndrome: Discontinue zolmitriptanif occurs (5.7, 7.4)
- Patients with Phenylketonuria: Zolmitriptan orally disintegrating tablet contains phenylalanine (5.9)
ADVERSE REACTIONS
Most common adverse reactions (≥5% and >placebo) were neck/throat/ jaw pain/tightness/pressure, dizziness, paresthesia, asthenia, somnolence, warm/cold sensation, nausea, heaviness sensation, and dry mouth (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Pregnancy: Based on animal data, may cause fetal harm (8.1) (8)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
2.2 Administration of Zolmitriptan Orally Disintegrating Tablets
2.3 Dosing in Patients with Hepatic Impairment
2.4 Dosing in Patients taking Cimetidine
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal Angina
5.2 Arrhythmias
5.3 Chest, Throat, Neck and Jaw Pain/Tightness/Pressure
5.4 Cerebrovascular Events
5.5 Other Vasospasm Reactions
5.6 Medication Overuse Headache
5.7 Serotonin Syndrome
5.8 Increase in Blood Pressure
5.9 Risks in Patients with Phenylketonuria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Ergot-containing Drugs
7.2 MAO-A Inhibitors
7.3 5-HT1B/1D Agonists
7.4 Selective Serotonin Reuptake Inhibitors and Serotonin Norepinephrine Reuptake Inhibitors
7.5 Cimetidine
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Zolmitriptan is indicated for the acute treatment of migraine with or without aura in adults.
Limitations of Use
- Only use zolmitriptan if a clear diagnosis of migraine has been established. If a patient has no response to zolmitriptan treatment for the first migraine attack, reconsider the diagnosis of migraine before zolmitriptan is administered to treat any subsequent attacks.
- Zolmitriptan is not indicated for the prevention of migraine attacks.
- Safety and effectiveness of zolmitriptan have not been established for cluster headache.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
The recommended starting dose of zolmitriptan tablet is 1.25 mg or 2.5 mg. The 1.25 mg dose can be achieved by manually breaking the functionally-scored 2.5 mg tablet in half. The maximum recommended single dose of zolmitriptan tablet is 5 mg.
In controlled clinical trials, a greater proportion of patients had headache response following a 2.5 mg or 5 mg dose than following a 1 mg dose. There was little added benefit from the 5 mg dose compared to the 2.5 mg dose, but adverse reactions were more frequent with the 5 mg dose.
If the migraine has not resolved by 2 hours after taking zolmitriptan, or returns after a transient improvement, a second dose may be administered at least 2 hours after the first dose. The maximum daily dose is 10 mg in any 24-hour period.
The safety of zolmitriptan in the treatment of an average of more than three migraines in a 30-day period has not been established.
2.2 Administration of Zolmitriptan Orally Disintegrating Tablets
Instruct patients not to break zolmitriptan orally disintegrating tablets because they are not functionally-scored. Administration with liquid is not necessary.
Orally disintegrating tablets are packaged in a blister pack. Instruct patients not to remove the tablet from the blister until just prior to dosing. Subsequently, instruct patients to peel the blister pack open, and to place the orally disintegrating tablet on the tongue, where it will dissolve and it will be swallowed with the saliva.
2.3 Dosing in Patients with Hepatic Impairment
The recommended dose of zolmitriptan tablet in patients with moderate to severe hepatic impairment is 1.25 mg (one-half of one 2.5 mg zolmitriptan tablet) because of increased zolmitriptan blood levels in these patients and elevation of blood pressure in some of these patients. Limit the total daily dose in patients with severe hepatic impairment to no more than 5 mg per day.
The use of zolmitriptan orally disintegrating tablets is not recommended in patients with moderate or severe hepatic impairment because these orally disintegrating tablets should not be broken in half [see Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)]. -
3 DOSAGE FORMS AND STRENGTHS
2.5 mg Orally Disintegrating Tablets: White to off-white, round, flat faced bevelled edge tablets debossed with '359' on one side and 'L' on other side.
5 mg Orally Disintegrating Tablets: White to off-white, round, flat faced bevelled edge tablets debossed with '360' on one side and 'L' on other side.
-
4 CONTRAINDICATIONS
Zolmitriptan is contraindicated in patients with:
- Ischemic coronary artery disease (angina pectoris, history of myocardial infarction, or documented silent ischemia), other significant underlying cardiovascular disease, or coronary artery vasospasm including Prinzmetal’s angina [see Warnings and Precautions (5.1)]
- Wolff-Parkinson-White Syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders [see Warnings and Precautions (5.2)]
- History of stroke, transient ischemic attack (TIA), or history of hemiplegic or basilar migraine because these patients are at a higher risk of stroke [see Warnings and Precautions (5.4)]
- Peripheral vascular disease (PVD) [see Warnings and Precautions (5.5)]
- Ischemic bowel disease [see Warnings and Precautions (5.5)]
- Uncontrolled hypertension [see Warnings and Precautions (5.8)]
- Recent use (i.e., within 24 hours) of another 5-HT1 agonist, ergotamine-containing medication, or ergot-type medication (such as dihydroergotamine or methysergide) [see Drug Interactions (7.1, 7.3)]
- Concurrent administration of a monoamine oxidase (MAO)-A inhibitor or recent use of a MAO-A inhibitor (that is within 2 weeks) [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)]
- Known hypersensitivity to zolmitriptan (angioedema and anaphylaxis seen) [see Adverse Reactions (6.2)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal Angina
Zolmitriptan is contraindicated in patients with ischemic or vasospastic coronary artery disease (CAD). There have been rare reports of serious cardiac adverse reactions, including acute myocardial infarction, occurring within a few hours following administration of zolmitriptan. Some of these reactions occurred in patients without known CAD. 5-HT1 agonists including zolmitriptan may cause coronary artery vasospasm (Prinzmetal Angina), even in patients without a history of CAD.
Perform a cardiovascular evaluation in triptan-naïve patients who have multiple cardiovascular risk factors (e.g., increased age, diabetes, hypertension, smoking, obesity, strong family history of CAD) prior to receiving zolmitriptan. Do not administer zolmitriptan if there is evidence of CAD or coronary artery vasospasm [see Contraindications (4)]. For patients with multiple cardiovascular risk factors who have a negative cardiovascular evaluation, consider administrating the first zolmitriptan dose in a medically-supervised setting and performing an electrocardiogram (ECG) immediately following zolmitriptan administration. For such patients, consider periodic cardiovascular evaluation in intermittent long-term users of zolmitriptan.5.2 Arrhythmias
Life-threatening disturbances of cardiac rhythm including ventricular tachycardia and ventricular fibrillation leading to death have been reported within a few hours following the administration of 5-HT1 agonists. Discontinue zolmitriptan if these disturbances occur. Zolmitriptan is contraindicated in patients with Wolff-Parkinson-White Syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders [see Contraindications (4)].
5.3 Chest, Throat, Neck and Jaw Pain/Tightness/Pressure
As with other 5-HT1 agonists, sensations of tightness, pain, and pressure in the chest, throat, neck, and jaw commonly occur after treatment with zolmitriptan and is usually non-cardiac in origin. However, perform a cardiac evaluation if these patients are at high cardiac risk. 5-HT1 agonists including zolmitriptan are contraindicated in patients with CAD or Prinzmetal’s variant angina [see Contraindications (4)].
5.4 Cerebrovascular Events
Cerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT1 agonists, and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the 5-HT1 agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine, when they were not.
As with other acute migraine therapies, before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with symptoms atypical for migraine, exclude other potentially serious neurological conditions. Zolmitriptan is contraindicated in patients with a history of stroke or transient ischemic attack [see Contraindications (4)].5.5 Other Vasospasm Reactions
5-HT1 agonists, including zolmitriptan, may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), splenic infarction, and Raynaud’s syndrome. In patients who experience symptoms or signs suggestive of a vasospastic reaction following the use of any 5-HT1 agonist, rule out a vasospastic reaction before receiving additional zolmitriptan doses[see Contraindications (4)].
Reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT1 agonists. Since visual disorders may be part of a migraine attack, a causal relationship between these events and the use of 5-HT1 agonists have not been clearly established.5.6 Medication Overuse Headache
Overuse of acute migraine drugs (e.g. ergotamine, triptans, opioids, or a combination of drugs for 10 or more days per month) may lead to exacerbation of headache (medication overuse headache). Medication overuse headache may present as migraine-like daily headaches or as a marked increase in frequency of migraine attacks. Detoxification of patients, including withdrawal of the overused drugs, and treatment of withdrawal symptoms (which often includes a transient worsening of headache) may be necessary.
5.7 Serotonin Syndrome
Serotonin syndrome may occur with triptans, including zolmitriptan, particularly during co-administration with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and MAO inhibitors [see Drug Interactions (7.5)]. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms usually rapidly occurs within minutes to hours of receiving a new or a greater dose of a serotonergic medication. Discontinue zolmitriptan if serotonin syndrome is suspected [see Drug Interactions (7.4)].
5.8 Increase in Blood Pressure
Significant elevations in systemic blood pressure have been reported in patients treated with 5-HT1 agonists including patients without a history of hypertension; very rarely, these increases in blood pressure have been associated with serious adverse reactions. In healthy subjects treated with 5 mg of zolmitriptan, an increase of 1 and 5 mm Hg in the systolic and diastolic blood pressure, respectively, was seen. In a study of patients with moderate to severe liver impairment, 7 of 27 patients experienced 20 to 80 mm Hg elevations in systolic and/or diastolic blood pressure after a dose of 10 mg of zolmitriptan.
As with all triptans, blood pressure should be monitored in zolmitriptan-treated patients. Zolmitriptan is contraindicated in patients with uncontrolled hypertension [see Contraindications (4)].
5.9 Risks in Patients with Phenylketonuria
Phenylalanine can be harmful to patients with phenylketonuria (PKU). Zolmitriptan orally disintegrating tablets contain phenylalanine (a component of aspartame). Each 2.5 mg and 5 mg orally disintegrating tablet contains 2.81 and 5.62 mg of phenylalanine, respectively.
-
6 ADVERSE REACTIONS
The following adverse reactions are described elsewhere in other sections of the prescribing information:
- Myocardial Ischemia, Myocardial Infarction, and Prinzmetal Angina
[seeWarnings and Precautions (5.1)].
- Arrthymias
[see Warnings and Precautions (5.2)].
- Chest and or Throat, Neck and Jaw Pain/Tightness/Pressure
[see Warnings and Precautions (5.3)].
- Cerebrovascular Events
[seeWarnings and Precautions (5.4)].
- Other Vasospasm Reactions
[seeWarnings and Precautions (5.5)].
- Medication Overuse Headache
[seeWarnings and Precautions (5.6)].
- Serotonin Syndrome
[seeWarnings and Precautions (5.7)].
- Increase in Blood Pressure
[seeWarnings and Precautions (5.8)].
- Risks in Patients with Phenylketonuria
[seeWarnings and Precautions (5.9)].
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In a long-term, open-label study where patients were allowed to treat multiple migraine attacks for up to 1 year, 8% (167 out of 2,058) withdrew from the trial because of adverse reaction.
The most common adverse reactions (≥5% and >placebo) in these trials were neck/throat/jaw pain, dizziness, paresthesia, asthenia, somnolence, warm/cold sensation, nausea, heaviness sensation, and dry mouth.
Table 1 lists the adverse reactions that occurred in ≥2% of the 2,074 patients in any one of the zolmitriptan 1 mg, 2.5 mg, or 5 mg dose groups in the controlled clinical trials of zolmitriptan in patients with migraines (Studies 1, 2, 3, 4, and 5) [see Clinical Studies (14)]. Only adverse reactions that were at least 2% more frequent in a zolmitriptan group compared to the placebo group are included.
Several of the adverse reactions appear dose related, notably paresthesia, sensation of heaviness or tightness in chest, neck, jaw, and throat, dizziness, somnolence and possibly asthenia and nausea.
Table 1: Adverse Reaction Incidence in Five Pooled Placebo-Controlled Migraine Clinical Trials1
Placebo
Zolmitriptan
Zolmitriptan
Zolmitriptan
1 mg
2.5 mg
5 mg
(n=401)
(n=163)
(n=498)
(n=1012)
ATYPICAL SENSATIONS
6%
12%
12%
18%
Paresthesia (all types)
2%
5%
7%
9%
Warm/cold sensation
4%
6%
5%
7%
PAIN AND PRESSURE SENSATIONS
7%
13%
14%
22%
Chest - pain/tightness/pressure and/or heaviness
1%
2%
3%
4%
Neck/throat/jaw - pain/tightness/pressure
3%
4%
7%
10%
Heaviness other than chest or neck
1%
1%
2%
5%
Other-Pressure/tightness/heaviness
0%
2%
2%
2%
DIGESTIVE
8%
11%
16%
14%
Dry mouth
2%
5%
3%
3%
Dyspepsia
1%
3%
2%
1%
Dysphagia
0%
0%
0%
2%
Nausea
4%
4%
9%
6%
NEUROLOGICAL
10%
11%
17%
21%
Dizziness
4%
6%
8%
10%
Somnolence
3%
5%
6%
8%
Vertigo
0%
0%
0%
2%
OTHER
Asthenia
3%
5%
3%
9%
Sweating
1%
0%
2%
3%
1Only adverse reactions that were at least 2% more frequent in a zolmitriptan group compared to the placebo group are included.
There were no differences in the incidence of adverse reactions in controlled clinical trials in the following subgroups: gender, weight, age, use of prophylactic medications, or presence of aura. There were insufficient data to assess the impact of race on the incidence of adverse reactions.
Less Common Adverse Reactions with Zolmitriptan Tablets:
In the paragraphs that follow, the frequencies of less commonly reported adverse clinical reactions are presented. Because the reports include reactions observed in open and uncontrolled studies, the role of zolmitriptan in their causation cannot be reliably determined. Furthermore, variability associated with adverse reaction reporting, the terminology used to describe adverse reactions, etc., limit the value of the quantitative frequency estimates provided. Adverse reaction frequencies were calculated as the number of patients who used zolmitriptan tablets and reported a reaction divided by the total number of patients exposed to zolmitriptan tablets (n=4,027). Reactions were further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: infrequent adverse reactions (those occurring in 1/100 to 1/1,000 patients) and rare adverse reactions (those occurring in less than 1/1,000 patients).
General: Infrequent were allergic reactions.
Cardiovascular: Infrequent were arrhythmias, hypertension, and syncope. Rare was tachycardia.
Neurological: Infrequent were agitation, anxiety, depression, emotional lability and insomnia; Rare were amnesia, hallucinations, and cerebral ischemia.
Skin: Infrequent were pruritus, rash and urticaria.
Urogenital: Infrequent were polyuria, urinary frequency and urinary urgency.
Adverse Reactions with Zolmitriptan Oral Disintegrating Tablets
The adverse reaction profile seen with zolmitriptan oral disintegrating tablets was similar to that seen with zolmitriptan tablets.
6.2 Postmarketing Experience
The following adverse reactions were identified during post approval use of zolmitriptan. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The reactions enumerated include all except those already listed in the Clinical Trials Experience section above or the Warnings and Precautions section.
Hypersensitivity Reactions:
As with other 5-HT1B/1D agonists, there have been reports of anaphylaxis, anaphylactoid, and hypersensitivity reactions including angioedema in patients receiving zolmitriptan. Zolmitriptan is contraindicated in patients with a history of hypersensitivity reaction to zolmitriptan.
-
7 DRUG INTERACTIONS
7.1 Ergot-containing Drugs
Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Because these effects may be additive, use of ergotamine-containing or ergot-type medications (like dihydroergotamine or methysergide) and zolmitriptan within 24 hours of each other is contraindicated [see Contraindications (4)].
7.2 MAO-A Inhibitors
MAO-A inhibitors increase the systemic exposure of zolmitriptan and its active N-desmethyl metabolite. Therefore, the use of zolmitriptan in patients receiving MAO-A inhibitors is contraindicated [see Contraindications (4) and Clinical Pharmacology (12.3)].
7.3 5-HT1B/1D Agonists
Concomitant use of other 5-HT1B/1D agonists (including triptans) within 24 hours of zolmitriptan treatment is contraindicated because the risk of vasospastic reactions may be additive [see Contraindications (4)].
7.4 Selective Serotonin Reuptake Inhibitors and Serotonin Norepinephrine Reuptake Inhibitors
Cases of life-threatening serotonin syndrome have been reported during co-administration of triptans and selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs) [see Warnings and Precautions (5.7)].
7.5 Cimetidine
Following administration of cimetidine, the half-life and blood levels of zolmitriptan and its active N-desmethyl metabolite were approximately doubled [see Clinical Pharmacology (12.3)]. If cimetidine and zolmitriptan are used concomitantly, limit the maximum single dose of zolmitriptan to 2.5 mg, not to exceed 5 mg in any 24-hour period [see Dosage and Administration, (2.4) and Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C: There are no adequate and well-controlled studies in pregnant women; therefore, zolmitriptan should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. In reproductive toxicity studies in rats and rabbits, oral administration of zolmitriptan to pregnant animals resulted in embryolethality and fetal abnormalities (malformations and variations) at clinically relevant exposures.
When zolmitriptan was administered to pregnant rats during the period of organogenesis at oral doses of 100, 400, and 1200 mg/kg/day (plasma exposures (AUCs) ≈280, 1100, and 5000 times the human AUC at the maximum recommended human dose (MRHD) of 10 mg/day), there was a dose-related increase in embryolethality. A no-effect dose for embryolethality was not established. When zolmitriptan was administered to pregnant rabbits during the period of organogenesis at oral doses of 3, 10, and 30 mg/kg/day (plasma AUCs ≈1, 11, and 42 times the human AUC at the MRHD), there were increases in embryolethality and in fetal malformations and variations. The no-effect dose for adverse effects on embryo-fetal development was associated with a plasma AUC similar to that in humans at the MRHD. When female rats were given zolmitriptan during gestation, parturition, and lactation at oral doses of 25, 100, and 400 mg/kg/day (plasma AUCs ≈70, 280, and 1100 times that in human at the MRHD), an increased incidence of hydronephrosis was found in the offspring. The no-effect dose was associated with a plasma AUC ≈280 times that in humans at the MRHD.8.3 Nursing Mothers
It is not known whether zolmitriptan is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from zolmitriptan, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. In rats, oral dosing with zolmitriptan resulted in levels in milk up to 4 times higher than in plasma.
8.4 Pediatric Use
The safety and effectiveness in pediatric patients have not been established. Therefore, zolmitriptan is not recommended for use in patients under 18 years of age.
One randomized, placebo-controlled clinical trial of zolmitriptan (2.5, 5 and 10 mg) evaluated 696 pediatric patients (aged 12 to 17 years) with migraines. This study did not demonstrate the efficacy of zolmitriptan compared to placebo in the treatment of migraine in adolescents. Adverse reactions in the adolescent patients treated with zolmitriptan were similar in nature and frequency to those reported in clinical trials in adults treated with zolmitriptan. Zolmitriptan has not been studied in pediatric patients less than 12 years old.
In the postmarketing experience with triptans, including zolmitriptan, there were no additional adverse reactions seen in pediatric patients that were not seen in adults.
8.5 Geriatric Use
Clinical studies of zolmitriptan did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
A cardiovascular evaluation is recommended for geriatric patients who have other cardiovascular risk factors (e.g., diabetes, hypertension, smoking, obesity, strong family history of coronary artery disease) prior to receiving zolmitriptan [see Warnings and Precautions (5.1)].
The pharmacokinetics of zolmitriptan were similar in geriatric patients (aged >65 years) compared to younger patients [see Clinical Pharmacology (12.3)].
8.6 Patients with Hepatic Impairment
After oral zolmitriptan administration, zolmitriptan blood levels were increased in patients with moderate to severe hepatic impairment, and significant elevation in blood pressure was observed in some of these patients [see Warnings and Precautions (5.8)]. Therefore, adjust the zolmitriptan tablet dose and administer with caution in patients with moderate or severe hepatic impairment [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
There is no experience with acute overdose of zolmitriptan. Clinical study subjects who received single 50 mg oral doses of zolmitriptan commonly experienced sedation.
There is no specific antidote to zolmitriptan. In cases of severe intoxication, intensive care procedures are recommended, including establishing and maintaining a patent airway, ensuring adequate oxygenation and ventilation, and monitoring and support of the cardiovascular system.
The elimination half-life of zolmitriptan is 3 hours [see Clinical Pharmacology (12.1)]; therefore, monitor patients after overdose with zolmitriptan for at least 15 hours or until symptoms or signs resolve. It is unknown what effect hemodialysis or peritoneal dialysis has on the plasma concentrations of zolmitriptan.
-
11 DESCRIPTION
Zolmitriptan orally disintegrating tablets contain zolmitriptan, which is a selective 5-hydroxytryptamine1B/1D (5-HT1B/1D) receptor agonist. Zolmitriptan is chemically designated as (S)-4-[[3-[2-(dimethylamino)ethyl]-1H-indol-5-yl]methyl]-2-oxazolidinone and has the following chemical structure:
The empirical formula is C16H21N3O2, representing a molecular weight of 287.36. Zolmitriptan is a white to off-white crystalline powder that is freely soluble in N,N-dimethyl formamide, dimethyl sulfoxide and practically insoluble in water.
Zolmitriptan orally disintegrating tablets are available as 2.5 mg and 5 mg white to off-white uncoated tablets. The orally disintegrating tablets contain mannitol, microcrystalline cellulose, crospovidone, aspartame [see Warnings and Precautions (5.9)], sodium stearyl fumarate, colloidal silicon dioxide, magnesium stearate, orange flavor, modified food starch and triacetin.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Zolmitriptan binds with high affinity to human recombinant 5-HT1D and 5-HT1B receptors, and moderate affinity for 5-HT1A receptors. The N-desmethyl metabolite also has high affinity for 5-HT1B/1D and moderate affinity for 5-HT1A receptors.
Migraines are likely due to local cranial vasodilatation and/or to the release of sensory neuropeptides (vasoactive intestinal peptide, substance P and calcitonin gene-related peptide) through nerve endings in the trigeminal system. The therapeutic activity of zolmitriptan for the treatment of migraine headache is thought to be due to the agonist effects at the 5-HT1B/1D receptors on intracranial blood vessels (including the arterio-venous anastomoses) and sensory nerves of the trigeminal system which result in cranial vessel constriction and inhibition of pro-inflammatory neuropeptide release.
12.3 Pharmacokinetics
Absorption, Distribution, Metabolism, and Excretion
Absorption
Zolmitriptan is well absorbed after oral administration for zolmitriptan orally disintegrating tablets. Zolmitriptan displays linear kinetics over the dose range of 2.5 to 50 mg.
The AUC and Cmax of zolmitriptan are similar following administration of zolmitriptan tablets and zolmitriptan orally disintegrating tablets, but the Tmax is somewhat later with zolmitriptan orally disintegrating tablets, with a median Tmax of 3 hours for zolmitriptan orally disintegrating tablet compared with 1.5 hours for the zolmitriptan tablet. The AUC, Cmax, and Tmax for the active N-desmethyl metabolite are similar for the two formulations.
During a moderate to severe migraine attack, mean AUC0-4 and Cmax for zolmitriptan, dosed as a zolmitriptan tablet, were decreased by 40% and 25%, respectively, and mean Tmax was delayed by one-half hour compared to the same patients during a migraine free period.
Food has no significant effect on the bioavailability of zolmitriptan. No accumulation occurred on multiple dosing.
Distribution
Mean absolute bioavailability is approximately 40%. The mean apparent volume of distribution is 7 L/kg. Plasma protein binding of zolmitriptan is 25% over the concentration range of 10 to 1000 ng/mL.
Metabolism
Zolmitriptan is converted to an active N-desmethyl metabolite; the metabolite concentrations are about two-thirds that of zolmitriptan. Because the 5HT1B/1D potency of the metabolite is 2 to 6 times that of the parent compound, the metabolite may contribute a substantial portion of the overall effect after zolmitriptan administration.
Excretion
Total radioactivity recovered in urine and feces was 65% and 30% of the administered dose, respectively. About 8% of the dose was recovered in the urine as unchanged zolmitriptan. Indole acetic acid metabolite accounted for 31% of the dose, followed by N-oxide (7%) and N-desmethyl (4%) metabolites. The indole acetic acid and N-oxide metabolites are inactive.
Mean total plasma clearance is 31.5 mL/min/kg, of which one-sixth is renal clearance. The renal clearance is greater than the glomerular filtration rate suggesting renal tubular secretion.
Special Populations
Hepatic Impairment
In patients with severe hepatic impairment, the mean Cmax, Tmax, and AUC0-∞ of zolmitriptan were increased 1.5-fold, 2-fold (2 vs. 4 hours), and 3-fold, respectively, compared to subjects with normal hepatic function. Seven out of 27 patients experienced 20 to 80 mm Hg elevations in systolic and/or diastolic blood pressure after a 10 mg zolmitriptan dose. Adjust the zolmitriptan dose in patients with moderate or severe hepatic impairment [see Dosage and Administration (2.3) and Use in Specific Populations (8.6)].
Renal Impairment
Clearance of zolmitriptan was reduced by 25% in patients with severe renal impairment (Clcr ≥ 5 ≤ 25 mL/min) compared to subjects with normal renal function (Clcr > = 70 mL/min); no significant change in clearance was observed in patients with moderate renal impairment (Clcr ≥ 26 ≤ 50 mL/min).
Age
Zolmitriptan pharmacokinetics in healthy elderly nonmigraineur volunteers (age 65 to 76 years) was similar to those in younger non-migraineur volunteers (age 18 to 39 years).
Sex
Mean plasma concentrations of zolmitriptan were up to 1.5-fold higher in females than males.
Race
Retrospective analysis of pharmacokinetic data between Japanese and Caucasians revealed no significant differences.
Hypertensive Patients
No differences in the pharmacokinetics of zolmitriptan or its effects on blood pressure were seen in mild to moderate hypertensive volunteers compared with normotensive controls.
Drug Interaction Studies
All drug interaction studies were performed in healthy volunteers using a single 10 mg dose of zolmitriptan and a single dose of the other drug except where otherwise noted.
MAO Inhibitors
Following one week of administration of moclobemide (150 mg twice daily), a specific MAO-A inhibitor, there was an increase of about 25% in both Cmax and AUC for zolmitriptan and a 3-fold increase in the Cmax and AUC of the active N-desmethyl metabolite of zolmitriptan. MAO inhibitors are contraindicated in zolmitriptan-treated patients [see Contraindications (4), Warnings and Precautions (5.7), and Drug Interactions (7.2, 7.4)].
Selegiline, a selective MAO-B inhibitor, at a dose of 10 mg/day for 1 week, had no effect on the pharmacokinetics of zolmitriptan and its metabolite.
Cimetidine
Following the administration of cimetidine, the half-life and AUC of zolmitriptan (5 mg dose), and its active metabolite, were approximately doubled [see Dosage and Administration (2.4), Drug Interactions (7.5)].
Fluoxetine
The pharmacokinetics of zolmitriptan, as well as its effect on blood pressure, were unaffected by 4 weeks of pretreatment with oral fluoxetine (20 mg/day).
Propranolol
Cmax and AUC of zolmitriptan were increased 1.5-fold after one week of dosing with propranolol (160 mg/day). Cmax and AUC of the N-desmethyl metabolite were reduced by 30% and 15%, respectively. There were no changes in blood pressure or pulse rate following administration of propranolol with zolmitriptan.
Acetaminophen
A single 1 gram dose of acetaminophen did not alter the pharmacokinetics of zolmitriptan and its N-desmethyl metabolite. However, zolmitriptan administration delayed the Tmax of acetaminophen by one hour.
Metoclopramide
A single 10 mg dose of metoclopramide had no effect on the pharmacokinetics of zolmitriptan or its metabolites.
Oral Contraceptives
Retrospective analysis of pharmacokinetic data across studies indicated that mean Cmax and AUC of zolmitriptan were increased by 30% and 50%, respectively, and Tmax was delayed by one-half hour in women taking oral contraceptives. The effect of zolmitriptan on the pharmacokinetics of oral contraceptives has not been studied.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Zolmitriptan was administered to mice and rats at doses up to 400 mg/kg/day. Mice were dosed for 85 weeks (males) and 92 weeks (females); rats were dosed for 101 weeks (males) and 86 weeks (females). There was no evidence of drug-induced tumors in mice at plasma exposures (AUC) up to approximately 700 times that in humans at the maximum recommended human dose (MRHD) of 10 mg/day. In rats, there was an increase in the incidence of thyroid follicular cell hyperplasia and thyroid follicular cell adenomas in male rats receiving 400 mg/kg/day. No increase in tumors was observed in rats at 100 mg/kg/day, a dose associated with a plasma AUC approximately 700 times that in humans at the MRHD.
Mutagenesis
Zolmitriptan was positive in an in vitro bacterial reverse mutation (Ames) assay and in an in vitro chromosomal aberration assay in human lymphocytes. Zolmitriptan was negative in an in vitro mammalian gene cell mutation (CHO/HGPRT) assay and in oral in vivo mouse micronucleus assays in mouse and rat.
Impairment of Fertility
Studies of male and female rats administered zolmitriptan prior to and during mating and up to implantation showed no impairment of fertility at oral doses up to 400 mg/kg/day. The plasma exposure (AUC) at this dose was approximately 3000 times that in humans at the MRHD.
-
14 CLINICAL STUDIES
Zolmitriptan Tablets
The efficacy of zolmitriptan tablets in the acute treatment of migraine headaches was demonstrated in five randomized, double-blind, placebo-controlled studies (Studies 1, 2, 3, 4, and 5), of which two utilized the 1 mg dose, two utilized the 2.5 mg dose and four utilized the 5 mg dose. In Study 1, patients treated their headaches in a clinic setting. In the other studies, patients treated their headaches as outpatients. In Study 4, patients who had previously used sumatriptan were excluded, whereas in the other studies no such exclusion was applied.
Patients enrolled in these 5 studies were predominantly female (82%) and Caucasian (97%) with a mean age of 40 years (range 12-65). Patients were instructed to treat a moderate to severe headache. Headache response, defined as a reduction in headache severity from moderate or severe pain to mild or no pain, was assessed at 1, 2, and, in most studies, 4 hours after dosing. Associated symptoms such as nausea, photophobia, and phonophobia were also assessed. Maintenance of response was assessed for up to 24 hours post-dose. A second dose of zolmitriptan tablets or other medication was allowed 2 to 24 hours after the initial treatment for persistent and recurrent headache. The frequency and time to use of these additional treatments were also recorded. In all studies, the effect of zolmitriptan tablet was compared to placebo in the treatment of a single migraine attack.In all five studies, the percentage of patients achieving headache response 2 hours after treatment was significantly greater among patients who received zolmitriptan tablets at all doses (except for the 1 mg dose in the smallest study) compared to those who received placebo. In Studies 1 and 3, there was a statistically significant greater percentage of patients with headache response at 2 hours in the higher dose groups (2.5 and/or 5 mg) compared to the 1 mg dose group. There were no statistically significant differences between the 2.5 and 5 mg dose groups (or of doses up to 20 mg) for the primary end point of headache response at 2 hours in any study. The results of these controlled clinical studies are summarized in Table 1.
Table 2 Percentage of Patients with Headache Response (Reduction in Headache Severity from Moderate or Severe Pain to Mild or No Headache) 2 Hours Following Treatment in Studies 1 through 5
Placebo Zolmitriptan tablets
1mg
Zolmitriptan tablets
2.5 mg
Zolmitriptan tablets
5 mg
Study 1a 16%
(n=19)27%
(n=22)NA 60%*#
(n=20)Study 2 19%
(n=88)NA NA 66%*
(n=179)Study 3 34%
(n=121)50%*
(n=140)65%*#
(n=260)67%*#
(n=245)
n=number of patients randomized
*p<0.05 in comparison with placebo.
#p<0.05 in comparison with 1 mg.
a Study 1 was the only study in which patients treated the headache in a clinic setting.
b Study 4 was the only study where patients were excluded who had previously used sumatriptan.
NA - not applicable
The estimated probability of achieving an initial headache response by 4 hours following treatment in pooled Studies 2, 3, and 5 is depicted in Figure 1.
Figure 1 Estimated Probability of Achieving Initial Headache Response (Reduction in Headache Severity from Moderate or Severe Pain to Mild or No Headache) Within 4 Hours of Treatment in Pooled Studies 2, 3, and 5*
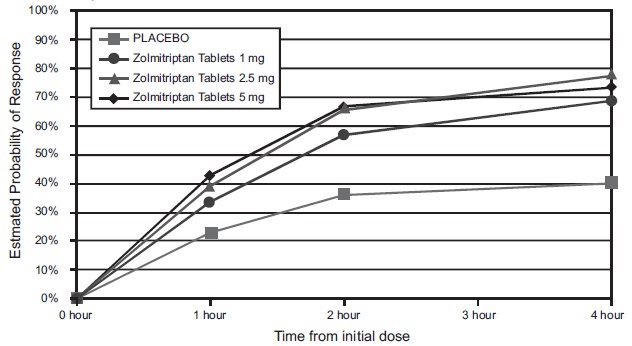
*In this Kaplan-Meier plot, the averages displayed are based on pooled data from 3 placebo controlled, outpatient trials. Patients not achieving headache response or taking additional treatment prior to 4 hours were censored at 4 hours.
For patients with migraine associated photophobia, phonophobia, and nausea at baseline, there was a decreased incidence of these symptoms following administration of zolmitriptan tablets as compared with placebo.
Two to 24 hours following the initial dose of study treatment, patients were allowed to use additional treatment for pain relief in the form of a second dose of study treatment or other medication. The estimated probability of patients taking a second dose or other medication for migraine over the 24 hours following the initial dose of study treatment is summarized in Figure 2.
Figure 2 The Estimated Probability Of Patients Taking A Second Dose Or Other Medication For Migraines Over The 24 Hours Following The Initial Dose Of Study Treatment in Pooled Studies 2, 3, and 5*
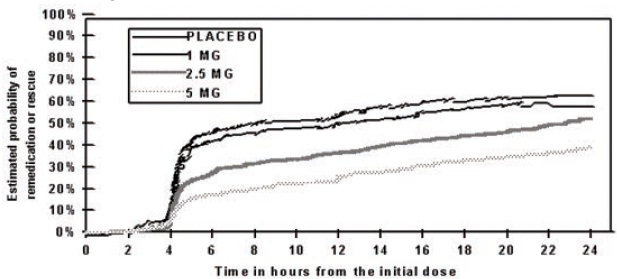
*In this Kaplan-Meier plot, patients not using additional treatments were censored at 24 hours. The plot includes both patients who had headache response at 2 hours and those who had no response to the initial dose. The studies did not allow taking additional doses of study medication within 2 hours post-dose.
The efficacy of zolmitriptan was unaffected by presence of aura; duration of headache prior to treatment; relationship to menses; gender, age, or weight of the patient; pre-treatment nausea or concomitant use of common migraine prophylactic drugs.
Zolmitriptan Orally Disintegrating Tablets
The efficacy of zolmitriptan 2.5 mg orally disintegrating tablets was demonstrated in a randomized, placebo-controlled trial (Study 6) that was similar in design to the trials of zolmitriptan tablets. Patients were instructed to treat a moderate to severe headache. Of the 471 patients treated in Study 6, 87% were female and 97% were Caucasian, with a mean age of 41 years (range 18 to 62).
At 2 hours post-dosing, there was a statistically significant greater percentage of patients treated with zolmitriptan orally disintegrating tablets 2.5 mg with a headache response (reduction in headache severity from moderate or severe pain to mild or no headache) compared to patients treated with placebo (63% vs. 22%). The estimated probability of achieving an initial headache response by 2 hours following treatment with zolmitriptan orally disintegrating tablets is depicted in Figure 3.
Figure 3 Estimated Probability of Achieving Initial Headache Response (Reduction in Headache Severity from Moderate or Severe Pain to Mild or No Headache) Within 2 Hours in Study 6*
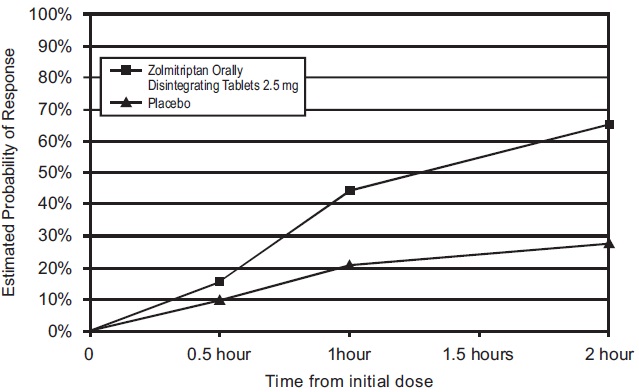
*In this Kaplan-Meier plot patients taking additional treatment or not achieving headache response prior to 2 hours were censored at 2 hours.
For patients with migraine-associated photophobia, phonophobia and nausea at baseline, there was a decreased incidence of these symptoms following administration of zolmitriptan orally disintegrating tablets as compared to placebo.
Two to 24 hours following the initial dose of study treatment, patients were allowed to use additional treatment in the form of a second dose of study treatment or other medication. The estimated probability of patients taking a second dose or other medication for migraine over the 24 hours following the initial dose of study treatment in Study 6 is summarized in Figure 4.
Figure 4 The Estimated Probability of Patients Taking a Second Dose or Other Medication for Migraines Over the 24 Hours Following The Initial Dose of Study Treatment in Study 6*
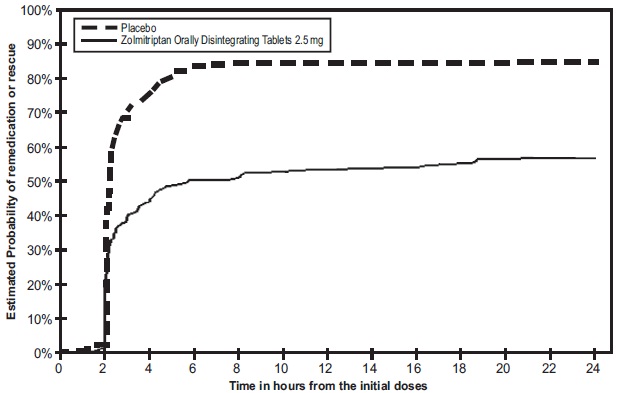
* In this Kaplan-Meier plot, patients not taking additional treatments were censored at 24 hours. The plot includes both patients who had headache response at 2 hours and those who had no response to the initial dose. Taking another dose of study medication was allowed 2 hours post-dose in Study 6. In contrast to studies of zolmitriptan tablets (Studies 1, 2, 3, 4, and 5), Study 6 allowed re-dosing of zolmitriptan oral disintegrating tablets prior to 4 hours.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
2.5 mg Orally Disintegrating Tablets -White to off-white, round, flat faced bevelled edge tablets debossed with ‘359’ on one side and ‘L’ on other side.
NDC: 46708-181-06 Carton of 1 blister of 6 tablets
5 mg Orally Disintegrating Tablets - White to off-white, round, flat faced bevelled edge tablets debossed with ‘360’ on one side and ‘L’ on other side.
NDC: 46708-182-03 Carton of 1 blister of 3 tablets
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Protect from light and moisture.
-
17 PATIENT COUNSELING INFORMATION
See FDA Approved Patient Labeling (Patient Information).
Myocardial Ischemia and/or Infarction, Prinzmetal’s angina, Other Vasospastic Reactions, and Cerebrovascular Events
Inform patients that zolmitriptan may cause serious cardiovascular adverse reactions such as myocardial infarction or stroke, which may result in hospitalization and even death. Although serious cardiovascular reactions can occur without warning symptoms, instruct patients to be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and instruct them to ask for medical advice when observing any indicative sign or symptoms. Instruct patients to seek medical advice if they have symptoms of other vasospastic reactions [see Warnings and Precautions (5.1, 5.2, 5.4, 5.5)].
Medication Overuse Headache
Inform patients that use of drugs to treat acute migraines for 10 or more days per month may lead to an exacerbation of headache, and encourage patients to record headache frequency and drug use (e.g., by keeping a headache diary) [see Warnings and Precautions (5.6)].
Serotonin Syndrome
Inform patients about the risk of serotonin syndrome with the use of zolmitriptan or other triptans,-particularly during combined use with selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs) [see Warnings and Precautions (5.7)].
Pregnancy
Inform patients that zolmitriptan should not be used during pregnancy unless the potential benefit justifies the potential risk to the fetus [see Use in Specific Populations (8.1)].
Nursing Mothers
Advise patients to notify their healthcare provider if they are breastfeeding or plan to breastfeed [see Use in Specific Populations (8.3)].
Handling of zolmitriptan orally Disintegrating Tablets
Inform patients not to break zolmitriptan oral disintegrating tablets. Inform patients that the orally disintegrating tablet is packaged in a blister. Instruct patients not to remove the oral disintegrating tablet from the blister until just prior to dosing. Instruct patients that prior to dosing, peel open the blister pack and place the orally disintegrating tablet on the tongue, where it will dissolve and be swallowed with the saliva [see Dosage and Administration (2.2)].
Patients with Phenylketonuria
Inform patients with phenylketonuria (PKU) that zolmitriptan orally disintegrating tablet contains phenylalanine (a component of aspartame) [see Warnings and Precautions (5.9)].
-
Patient Information
Zolmitriptan (ZOLE-mi-TRIP-tan) Orally Disintegrating Tablets
Please read this information before you start taking zolmitriptan and each time you renew your prescription just in case anything has changed. Remember, this summary does not take the place of discussions with your doctor. You and your doctor should discuss zolmitriptan when you start taking your medication and at regular checkups.
What is zolmitriptan?
Zolmitriptan is a prescription medication used to treat migraine headaches in adults. Zolmitriptan is not for other types of headaches. The safety and efficacy of zolmitriptan in patients under 18 have not been established.What is a Migraine Headache?
Migraine is an intense, throbbing headache. You may have pain on one or both sides of your head. You may have nausea and vomiting, and be sensitive to light and noise. The pain and symptoms of a migraine headache can be worse than a common headache. Some women get migraines around the time of their menstrual period. Some people have visual symptoms before the headache, such as flashing lights or wavy lines, called an aura.How does zolmitriptan work?
Treatment with zolmitriptan reduces swelling of blood vessels surrounding the brain. This swelling is associated with the headache pain of a migraine attack. Zolmitriptan blocks the release of substances from nerve endings that cause more pain and other symptoms like nausea, and sensitivity to light and sound. It is thought that these actions contribute to relief of your symptoms by zolmitriptan.Who should not take zolmitriptan?
Do not take zolmitriptan if you:
Have heart disease or a history of heart disease
Have uncontrolled high blood pressure
Have hemiplegic or basilar migraine (if you are not sure about this, ask your doctor)
Have or had a stroke or problems with your blood circulation
Have serious liver problems
Have taken any of the following medicines in the last 24 hours: other “triptans” like almotriptan (AXERT®), eletriptan (RELPAX®), frovatriptan (FROVA®), naratriptan (AMERGE®), rizatriptan (MAXALT®), sumatriptan (IMITREX®), sumatriptan/naproxen (TREXIMET); ergotamines like BELLERGAL-S®, CAFERGOT®, ERGOMAR®, WIGRAINE®; dihydroergotamine like D.H.E. 45® or MIGRANAL®; or methysergide (SANSERT®). These medications have side effects similar to zolmitriptan.
Have taken monoamine oxidase (MAO) inhibitors such as phenelzine sulfate (NARDIL®) or tranylcypromine sulfate (PARNATE®) for depression or other conditions within the last 2 weeks.
Are allergic to zolmitriptan or any of its ingredients. The active ingredient is zolmitriptan. The inactive ingredients are listed at the end of this leaflet.Tell your doctor about all the medicines you take or plan to take, including prescription and non-prescription medicines, supplements, and herbal remedies.
Tell your doctor if you are sensitive to phenylalanine, which can be found in the artificial sweetener aspartame. Zolmitriptan orally disintegrating tablet contains phenylalanine.Tell your doctor if you are taking selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs), two types of drugs for depression or other disorders. Common SSRIs are CELEXA® (citalopram HBr), LEXAPRO® (escitalopram oxalate), PAXIL® (paroxetine), PROZAC® (fluoxetine), SYMBYAX® (olanzapine/fluoxetine), ZOLOFT® (sertraline), SARAFEM® (fluoxetine) and LUVOX® (fluvoxamine). Common SNRIs are CYMBALTA® (duloxetine) and EFFEXOR® (venlafaxine). Your doctor will decide if you can take zolmitriptan with your other medicines.
Tell your doctor if you know that you have any of the following: risk factors for heart disease like high cholesterol, diabetes, smoking, obesity (overweight), menopause, or a family history of heart disease or stroke.
Tell your doctor if you are pregnant, planning to become pregnant, breast feeding, planning to breast feed, or not using effective birth control.
How should I take zolmitriptan?
Take zolmitriptan exactly as your doctor tells you to take it. Your doctor will tell you how much zolmitriptan to take and when to take it.
If you take zolmitriptan oral disintegrating tablets, do not remove the tablet from the blister pack until you are ready to take your medicine.
You do not need to take any liquids with your zolmitriptan oral disintegrating tablets.
Take zolmitriptan oral disintegrating tablets whole.
Place zolmitriptan oral disintegrating tablets on your tongue, where it will dissolve.
Safely throw away any unused tablets or pieces of tablets that have been removed from the blister packaging.
If your headache comes back after your first dose, you may take a second dose anytime after 2 hours of taking the first dose. For any attack where the first dose did not work, do not take a second dose without talking with your doctor. Do not take more than a total of 10 mg of zolmitriptan (tablets or spray combined in any 24 hour period). If you take too much medicine, contact your doctor, hospital emergency department, or poison control center right away.What are the possible side effects of zolmitriptan?
Zolmitriptan is generally well tolerated. As with any medicine, people taking zolmitriptan may have side effects. The side effects are usually mild and do not last long.The most common side effects of zolmitriptan are:
Pain, pressure or tightness in the neck, throat or jaw
dizziness
tingling or other abnormal sensations
tiredness
drowsiness
feeling warm or cold
nausea
feeling of tightness or heaviness in other areas of the body
dry mouth
In very rare cases, patients taking triptans may experience serious side effects, such as heart attacks, high blood pressure, stroke, or serious allergic reactions. Extremely rarely, patients have died. Call your doctor right away if you have any of the following problems after taking zolmitriptan:severe tightness, pain, pressure or heaviness in your chest, throat, neck, or jaw
shortness of breath or wheezing
sudden or severe stomach pain
hives; tongue, mouth, or throat swelling
problems seeing
unusual weakness or numbnessSome people may have a reaction called serotonin syndrome, which can be life-threatening, when they use zolmitriptan. In particular, this reaction may occur when they use zolmitriptan together with certain types of antidepressants known as SSRIs or SNRIs. Symptoms may include mental changes (hallucinations, agitation, coma), fast heartbeat, changes in blood pressure, high body temperature or sweating, tight muscles, trouble walking, nausea, vomiting, and diarrhea. Call your doctor immediately if you have any of these symptoms after taking zolmitriptan.
Call your doctor for medical advice about side effects. You may report side effects at 1-866-562-4597 or FDA at 1-800-FDA-1088.
This is not a complete list of side effects. Talk to your doctor if you develop any symptoms that concern you.
What to do in case of an overdose?
Call your doctor or poison control center or go to the nearest hospital emergency room.General advice about zolmitriptan
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use zolmitriptan for a condition for which it was not prescribed. Do not give zolmitriptan to other people, even if they have the same symptoms as you. People may be harmed if they take medicines that have not been prescribed for them.This leaflet summarizes the most important information about zolmitriptan. If you would like more information about zolmitriptan, talk to your doctor. You can ask your doctor or pharmacist for information on zolmitriptan that is written for healthcare professionals.
What are the Ingredients in zolmitriptan?
Zolmitriptan Orally Disintegrating Tablets
Active ingredient: zolmitriptan
Inactive ingredients: mannitol, microcrystalline cellulose, crospovidone, aspartame, sodium stearyl fumarate, colloidal silicon dioxide, magnesium stearate, orange flavor, modified food starch and triacetin.Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature] and away from children. Protect from light and moisture. Discard when expired.
Other brands mentioned are trademarks of their respective owners. The makers of these brands are not affiliated with Alembic Pharmaceuticals Limited or its products.
Manufactured by:
Alembic Pharmaceuticals Limited
(Formulation Division),
Village Panelav, P. O. Tajpura, Near Baska,
Taluka-Halol, Panchmahal, Gujarat, India.Revised: 03/2016
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 2.5 mg
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 5mg
-
INGREDIENTS AND APPEARANCE
ZOLMITRIPTAN
zolmitriptan tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 46708-181 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZOLMITRIPTAN (UNII: 2FS66TH3YW) (ZOLMITRIPTAN - UNII:2FS66TH3YW) ZOLMITRIPTAN 2.5 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE (UNII: 68401960MK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ASPARTAME (UNII: Z0H242BBR1) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) MAGNESIUM STEARATE (UNII: 70097M6I30) TRIACETIN (UNII: XHX3C3X673) ORANGE (UNII: 5EVU04N5QU) Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND (flat faced bevelled edge) Size 6mm Flavor ORANGE Imprint Code 359;L Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46708-181-16 6 in 1 CARTON 12/01/2016 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205074 12/01/2016 ZOLMITRIPTAN
zolmitriptan tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 46708-182 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZOLMITRIPTAN (UNII: 2FS66TH3YW) (ZOLMITRIPTAN - UNII:2FS66TH3YW) ZOLMITRIPTAN 5 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSPOVIDONE (UNII: 68401960MK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ASPARTAME (UNII: Z0H242BBR1) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) MAGNESIUM STEARATE (UNII: 70097M6I30) ORANGE (UNII: 5EVU04N5QU) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND (flat faced bevelled edge) Size 9mm Flavor ORANGE Imprint Code 360;L Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46708-182-13 3 in 1 CARTON 12/01/2016 1 3 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205074 12/01/2016 Labeler - Alembic Pharmaceuticals Limited (650574663) Establishment Name Address ID/FEI Business Operations Alembic Pharmaceuticals Limited 650574671 MANUFACTURE(46708-181, 46708-182)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.