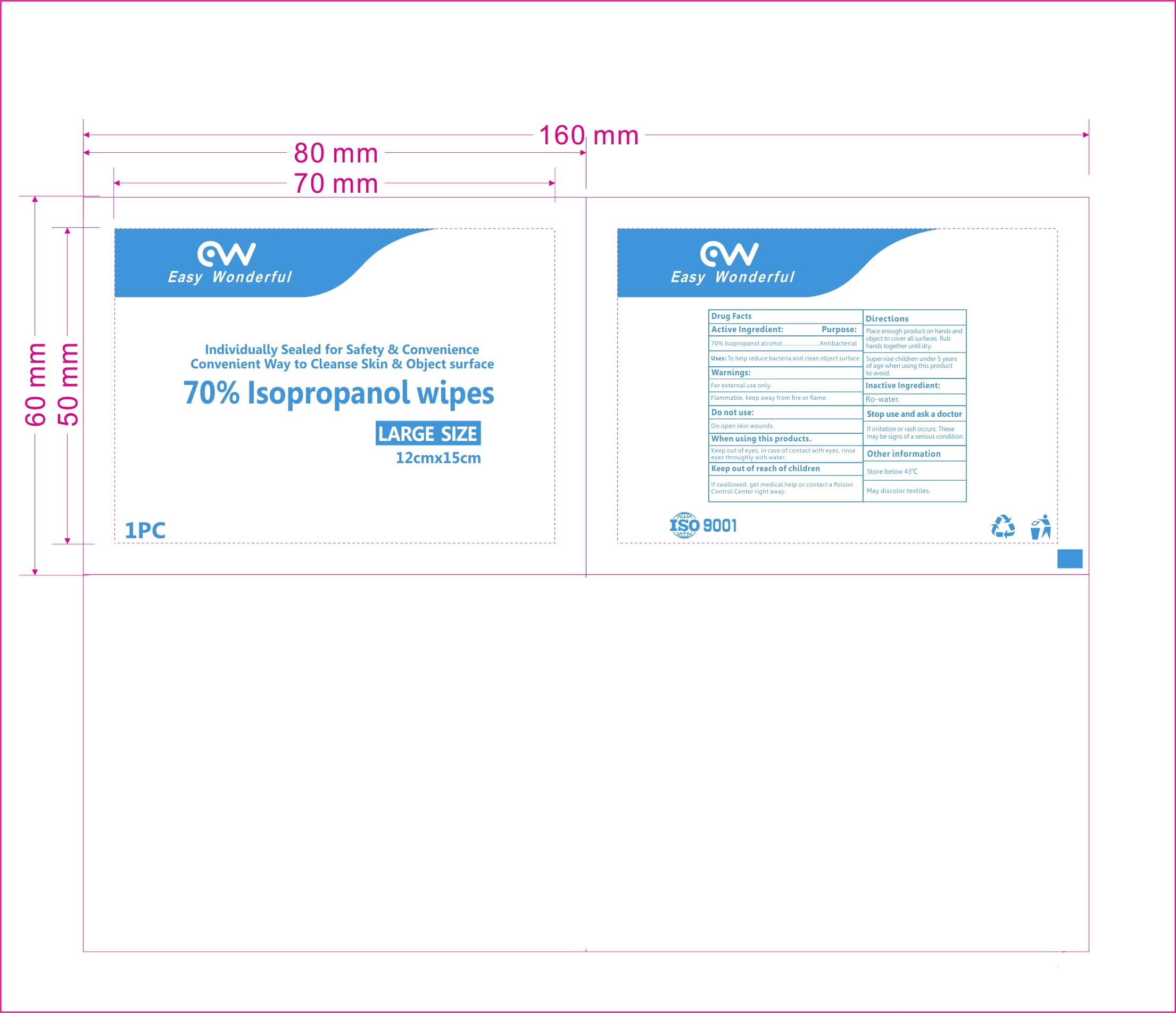
77827-101-01 70% Isopropanol wipes
70% Isopropanol wipes by
Drug Labeling and Warnings
70% Isopropanol wipes by is a Otc medication manufactured, distributed, or labeled by Tianjin Lantian Bishui Technology Co,. Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
70% ISOPROPANOL WIPES- isopropyl alcohol cloth
Tianjin Lantian Bishui Technology Co,. Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
77827-101-01 70% Isopropanol wipes
When using this products. Keep out of eyes, in case of contact with eyes, rinse eyes thoroughly with water.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
| 70% ISOPROPANOL WIPES
isopropyl alcohol cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Tianjin Lantian Bishui Technology Co,. Ltd. (550851452) |
| Registrant - Tianjin Lantian Bishui Technology Co,. Ltd. (550851452) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Tianjin Lantian Bishui Technology Co,. Ltd. | 550851452 | manufacture(77827-101) | |
Revised: 4/2023
Document Id: f9ca47fb-1ec0-0719-e053-6294a90a6d2f
Set id: b8eeb9e5-c396-b399-e053-2a95a90a5e4f
Version: 3
Effective Time: 20230420
Tia
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.