NEPHRAMINE- histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine, cysteine, and sodium bisulfite injection, solution
NephrAmine by
Drug Labeling and Warnings
NephrAmine by is a Prescription medication manufactured, distributed, or labeled by B. Braun Medical Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
5.4% NephrAmine® (Essential Amino Acid Injection) is a sterile, nonpyrogenic solution containing crystalline essential amino acids plus histidine. Each 250 mL unit provides Rose's recommended daily intake of essential amino acids1 plus 625 mg of histidine, considered essential for uremics. The total nitrogen content of a 250 mL unit is approximately 1.6 grams (10 g of protein equivalent) in 14 grams of amino acids. All amino acids designated USP are the "L" isomer.
Each 100 mL contains:
Histidine USP2..............................................0.25 g
Isoleucine USP..............................................0.56 g
Leucine USP.................................................0.88 g
Lysine...........................................................0.64 g
(added as Lysine Acetate USP....................0.90 g)
Methionine USP............................................0.88 g
Phenylalanine USP.........................................0.88 g
Threonine USP..............................................0.40 g
Tryptophan USP...........................................0.20 g
Valine USP....................................................0.64 g
Cysteine....................................................<0.014 g
(as Cysteine HClH2O USP....................<0.020 g)
Sodium Bisulfite (as an antioxidant)..............<0.05 g
Water for Injection USP......................................qs
pH adjusted with Sodium Hydroxide NF as required.
pH: 6.5 (6.0–7.0); Calculated Osmolarity: 435 mOsmol/liter
Total Nitrogen: Approx. 0.65 g/100 mL
Concentration of Electrolytes (mEq/liter): Sodium 5
Chloride <3; Acetate Approx. 44
- 1 Rose WC: The sequence of events leading to the establishment of the amino acid needs of man. Am J Public Health; 1968: 58 (11):2020–2027.
- 2 Histidine is considered an essential amino acid in uremic patients.
-
CLINICAL PHARMACOLOGY
NephrAmine® provides an intravenously compatible mixture of essential amino acids which, when infused with hypertonic dextrose as a source of calories, plus electrolytes, minerals, and vitamins, provides in a small volume of fluid all ingredients (with the exception of essential fatty acids) needed for total parenteral nutrition in patients with renal disease.
Infusion of NephrAmine® and hypertonic dextrose provides essential amino acids and calories for protein synthesis to promote improved cellular metabolic balance. Infusion of these components can decrease the rate of rise of blood urea nitrogen and minimize deterioration of serum potassium, magnesium, and phosphorus balance in patients with impaired renal function. The extent to which essential amino acids and calories promote incorporation of waste urea nitrogen into newly synthesized amino acids in man, as it does in experimental animals, is, so far, not established.
The accelerated decrease in serum creatinine levels seen in patients with limited extra-renal complications suggests that treatment with NephrAmine® and hypertonic dextrose leads to earlier return of renal function in patients with potentially reversible acute renal failure. By providing nutritional support and promoting biochemical improvement as well as earlier return of renal function, NephrAmine® and hypertonic dextrose decrease morbidity associated with acute renal failure.
It is thought that acetate from lysine acetate, under the condition of parenteral nutrition, does not impact net acid-base balance when renal and respiratory functions are normal. Clinical evidence seems to support this thinking; however, confirmatory experimental evidence is not available.
The amounts of sodium and chloride present are not of clinical significance.
-
INDICATIONS AND USAGE
5.4% NephrAmine® (Essential Amino Acid Injection) is indicated for adult and pediatric use, in conjunction with other measures, to provide nutritional support for uremic patients, particularly when oral nutrition is infeasible or impractical. See WARNINGS, PRECAUTIONS, Pediatric Use, Special Precautions in Pediatric Patients, and DOSAGE AND ADMINISTRATION.
- CONTRAINDICATIONS
-
WARNINGS
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
This product contains sodium bisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
Safe and effective use of central venous nutrition requires a knowledge of nutrition as well as clinical expertise in recognition and treatment of the complications which can occur. Frequent clinical evaluation and laboratory determinations are necessary for proper monitoring of central venous nutrition. Laboratory tests should include measurement of blood sugar, electrolyte, and serum protein concentrations; kidney and liver function tests; and evaluation of acid-base balance and fluid balance. Other laboratory tests may be suggested by the patient's condition.
NephrAmine® does not replace dialysis and conventional supportive therapy in patients with renal failure.
Administration of NephrAmine® to pediatric patients, especially in high dose ranges, may result in hyperammonemia. Administration of NephrAmine® to infants, particularly neonates and low birth weight infants, may result in elevated plasma amino acid levels (e.g., hypermethionemia) and hyperammonemia. In these very young age groups, amino acid formulations developed specifically for nutritional support of infants and pediatric patients, should be considered.
Clinically significant hypokalemia, hypophosphatemia, or hypomagnesemia may occur as a result of therapy with NephrAmine® and hypertonic dextrose and replacement therapy may become necessary.
Administration of nitrogen in any form to patients with marked hepatic insufficiency or hepatic coma may result in plasma amino acid imbalances, hyperammonemia, or central nervous system deterioration. NephrAmine® should, therefore, be used with caution in such patients.
The intravenous administration of these solutions can cause fluid and/or solute overload resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema. The risk of dilutional states is inversely proportional to the solute concentration of the solution infused. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the concentration of the solution.
Conservative doses of amino acids should be given, dictated by the nutritional status of the patient.
-
PRECAUTIONS
General
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation. Significant deviations from normal concentrations may require the use of additional electrolyte supplements.
In order to promote urea nitrogen reutilization in patients with renal failure, it is essential to provide adequate calories with minimal amounts of the essential amino acids, and to severely restrict the intake of nonessential nitrogen. Hypertonic dextrose solutions are a convenient and metabolically effective source of concentrated calories.
Fluid balance must be carefully monitored in patients with renal failure and care should be taken to avoid circulatory overload, particularly in association with cardiac insufficiency.
In patients with myocardial infarct, infusion of amino acids should always be accompanied by dextrose, since in anoxia, free fatty acids cannot be utilized by the myocardium, and energy must be produced anaerobically from glycogen or glucose.
Strongly hypertonic nutrient solutions should be administered through an indwelling intravenous catheter with the tip located in the superior vena cava.
Special care must be taken when giving hypertonic dextrose to glucose-intolerant patients such as diabetic or prediabetic and uremic patients, especially when the latter are receiving peritoneal dialysis. To prevent severe hyperglycemia in such patients, insulin may be required.
Administration of glucose at a rate exceeding the patient's utilization may lead to hyperglycemia, coma, and death.
Administration of amino acids without carbohydrates may result in the accumulation of ketone bodies in the blood. Correction of this ketonemia may be achieved by the administration of carbohydrates.
Abrupt cessation of hypertonic dextrose infusion may result in rebound hypoglycemia.
When 5.4% NephrAmine® (Essential Amino Acid Injection) is subjected to changes in temperature, there is a chance that some transient crystallization of amino acids may occur. Thorough shaking of the bottle for about one minute should redissolve the amino acids. If the amino acids do not completely redissolve, the bottle must be rejected.
To minimize the risk of possible incompatibilities arising from mixing this solution with other additives that may be prescribed, the final infusate should be inspected for cloudiness or precipitation immediately after mixing, prior to administration, and periodically during administration.
Use only if solution is clear and vacuum is present.
Drug product contains no more than 25 µg/L of aluminum.
Laboratory Tests
Frequent clinical evaluation and laboratory determinations are necessary for proper monitoring during administration.
Laboratory tests should include measurement of blood sugar, electrolyte, and serum protein concentrations, kidney and liver function tests, and evaluation of acid-base balance and fluid balance. Other laboratory tests may be suggested by the patient's condition.
Drug Interactions
Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No in vitro or in vivo carcinogenesis, mutagenesis, or fertility studies have been conducted with 5.4% NephrAmine® (Essential Amino Acid Injection).
Pregnancy
Teratogenic Effects – Pregnancy Category C
Animal reproduction studies have not been conducted with 5.4% NephrAmine® (Essential Amino Acid Injection). It is also not known whether NephrAmine® can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. NephrAmine® should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when NephrAmine® is administered to a nursing woman.
Pediatric Use
Safety and effectiveness of amino acid injections in pediatric patients have not been established by adequate and well-controlled studies. However, the use of amino acid injections in pediatric patients as an adjunct in the offsetting of nitrogen loss or in the treatment of negative nitrogen balance is well established in the medical literature. In pediatric patients, the final solution should not exceed twice normal serum osmolarity (718 mOsmol/L). See INDICATIONS AND USAGE, WARNINGS, and DOSAGE AND ADMINISTRATION.
Geriatric Use
NephrAmine® has not been studied in geriatric patients. Elderly patients are known to be more prone to fluid overload and electrolyte imbalance than younger patients. This may be related to impairment of renal function which is more frequent in an elderly population. As a result the need for careful monitoring of fluid and electrolyte therapy is greater in the elderly. All patients, including the elderly, require an individual dose of all parenteral nutrition products to be determined by the physician on an individual case-by-case basis, which will be based on body weight, clinical condition and the results of laboratory monitoring tests. There is no specific geriatric dose.
See WARNINGS.
Special Precautions for Central Venous Nutrition
Administration by central venous catheter should be used only by those familiar with this technique and its complications.
Central venous nutrition may be associated with complications which can be prevented or minimized by careful attention to all aspects of the procedure including solution preparation, administration, and patient monitoring. It is essential that a carefully prepared protocol, based on current medical practices, be followed, preferably by an experienced team.
Although a detailed discussion of the complications of central venous nutrition is beyond the scope of this insert, the following summary lists those based on current literature:
Technical
The placement of a central venous catheter should be regarded as a surgical procedure. One should be fully acquainted with various techniques of catheter insertion as well as recognition and treatment of complications. For details of techniques and placement sites, consult the medical literature. X-ray is the best means of verifying catheter placement. Complications known to occur from the placement of central venous catheters are pneumothorax, hemothorax, hydrothorax, artery puncture and transection, injury to the brachial plexus, malposition of the catheter, formation of arterio-venous fistula, phlebitis, thrombosis, and air and catheter embolus.
Septic
The constant risk of sepsis is present during central venous nutrition. Since contaminated solutions and infusion catheters are potential sources of infection, it is imperative that the preparation of parenteral nutrition solutions and the placement and care of catheters be accomplished under controlled aseptic conditions.
Parenteral nutrition solutions should ideally be prepared in the hospital pharmacy under a laminar flow hood. The key factor in their preparation is careful aseptic technique to avoid inadvertent touch contamination during mixing of solutions and subsequent admixtures.
Parenteral nutrition solutions should be used promptly after mixing. Any storage should be under refrigeration for as brief a time as possible. Administration time for a single bottle and set should never exceed 24 hours.
Consult the medical literature for a discussion of the management of sepsis during central venous nutrition. In brief, typical management includes replacing the solution being administered with a fresh container and set, and the remaining contents are cultured for bacterial or fungal contamination. If sepsis persists and another source of infection is not identified, the catheter is removed, the proximal tip cultured, and a new catheter reinserted when the fever has subsided. Nonspecific, prophylactic antibiotic treatment is not recommended. Clinical experience indicates that the catheter is likely to be the prime source of infection as opposed to aseptically prepared and properly stored solutions.
Metabolic
The following metabolic complications have been reported: metabolic acidosis, hypophosphatemia, alkalosis, hyperglycemia and glycosuria, osmotic diuresis and dehydration, rebound hypoglycemia, elevated liver enzymes, hypo- and hypervitaminosis, electrolyte imbalances, and elevated plasma amino acid levels and hyperammonemia in infants and pediatric patients. Frequent clinical evaluation and laboratory determinations are necessary, especially during the first few days of central venous nutrition, to prevent or minimize these complications.
Special Precautions in Patients with Renal Insufficiency
Frequent laboratory studies are necessary in patients with renal insufficiency due to underlying metabolic abnormalities. Hyperglycemia, a frequent complication, may not be reflected by glycosuria in renal failure. Blood glucose, therefore, must be determined frequently, often every six hours to guide dosage of dextrose and insulin if required.
Serum concentrations of potassium, phosphorus, and magnesium may dramatically decline with successful treatment, individually or together; these substances should be supplemented as required. Special care must be taken to avoid hypokalemia in digitalized patients, or those with cardiac arrhythmias.
Special Precautions in Pediatric Patients
5.4% NephrAmine® (Essential Amino Acid Injection) should be used with special caution in pediatric patients, due to limited clinical experience.
Laboratory and clinical monitoring of pediatric patients, especially when nutritionally depleted, must be extensive and frequent. Initial total daily dose should be low, and increased slowly. Dosage of NephrAmine® above one gram of essential amino acids per kilogram body weight per day is not recommended.
For infants, especially neonates and low birth weight infants, amino acid formulations developed specifically for nutritional support of infants and pediatric patients should be considered. If NephrAmine® is administered to these very young patients, extra caution in frequent monitoring of plasma amino acid levels and serum ammonia is strongly recommended.
Frequent monitoring of blood glucose is required in neonates, low birth-weight, or septic infants as infusion of hypertonic dextrose carries a greater risk of hyperglycemia in such patients.
The absence of arginine in NephrAmine® may accentuate the risk of hyperammonemia in infants.
In pediatric patients, the final solution should not exceed twice normal serum osmolarity (718 mOsmol/L).
-
ADVERSE REACTIONS
See WARNINGS and Special Precautions for Central Venous Nutrition.
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis, and hypervolemia.
Symptoms may result from an excess or deficit of one or more of the ions present in the solution infused, therefore, frequent monitoring of electrolyte levels is essential.
Infrequent instances of hyperammonemia have been reported following administration of essential amino acid solutions to patients with massive gastrointestinal hemorrhage, nonuremic infants and pediatric patients or following administration of higher than recommended doses to adult or pediatric patients. Elevated plasma amino acid levels (hypermethionemia) have also been reported in infants especially in higher dosage ranges. Elevated serum ammonia levels, plasma amino acid levels, and clinical symptoms may subside when the infusions are discontinued.
Phosphorus deficiency may lead to impaired tissue oxygenation and acute hemolytic anemia. Relative to calcium, excessive phosphorus intake can precipitate hypocalcemia with cramps, tetany, and muscular hyperexcitability.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
The objective of nutritional management of renal decompensation is the provision of sufficient amino acid and caloric support for protein synthesis without greatly exceeding the renal capacity to excrete metabolic wastes.
Three grams of nitrogen per day provided as essential amino acids with adequate calories produce nitrogen equilibrium in many stable patients with chronic uremia. Although nitrogen requirements may be higher in stressed or acutely uremic patients, or those on dialysis, provision of additional nitrogen may not be possible due to fluid intake limits or glucose intolerance.
The usual methods of determining individual patient requirements for amino acids such as nitrogen balance or daily body weight are difficult to perform or interpret in the uremic patient. Therefore, dosage is guided by the patient's fluid intake limits and glucose and nitrogen tolerances, as well as metabolic and clinical response. Rate of rise of blood urea nitrogen generally diminishes with infusion of essential amino acids. However, excessive intake of dietary protein or increased protein catabolism may alter this response.
Adult Use
Generally, 250 to 500 mL of 5.4% NephrAmine® (Essential Amino Acid Injection), containing approximately 1.6 to 3.2 grams of nitrogen (in 13.4 to 26.8 grams of essential amino acids), are given daily. Adequate calories should be provided simultaneously. Each 250 mL of NephrAmine® is typically mixed aseptically with 500 mL of 70% dextrose to yield a solution of 1.8% NephrAmine® in 47% dextrose. This mixture provides a calorie-to-nitrogen ratio of 744:1.
Solution administrated by peripheral vein should not exceed twice normal serum osmolarity (718 mOsmol/L).
Pediatric Use
Initial total daily dose should be low and increased slowly. As the dose is increased, frequent laboratory and clinical monitoring is strongly recommended, especially in very young patients, to avoid clinically significant elevations of serum ammonia and plasma amino acid levels. Dosage of NephrAmine® above one gram of essential amino acids per kg of body weight per day is not recommended. In pediatric patients, the final solution should not exceed twice normal serum osmolarity (718 mOsmol/L).
Use of 5.4% NephrAmine® in pediatric patients is governed by the same considerations that affect the use of any amino acid solution in pediatrics. The amount administered is dosed on the basis of grams of amino acids/kg of body weight/day.
See INDICATIONS AND USAGE, WARNINGS, PRECAUTIONS, Pediatric Use, and Special Precautions in Pediatric Patients.
Fat emulsion coadministration should be considered when prolonged (more than 5 days) parenteral nutrition is required in order to prevent essential fatty acid deficiency (E.F.A.D.). Serum lipids should be monitored for evidence of E.F.A.D. in patients maintained on fat free TPN.
Electrolyte supplementation may be required. Undiluted NephrAmine® (Essential Amino Acid Injection) contains 5 mEq/liter of sodium. Elevated serum potassium, phosphorus, and magnesium levels generally decrease during treatment with NephrAmine®. Although these effects are beneficial, especially in acute renal failure, in some instances the reduction may be so great that supplementation of these electrolytes is required, especially in the presence of cardiac arrhythmias or digitalis toxicity. During periods of anuria or oliguria, electrolyte supplementation should be done with caution, even if serum levels are in the low normal range.
Compatibility of electrolyte additives to the 5.4% NephrAmine®/hypertonic dextrose mixture must be considered, and potentially incompatible ions such as calcium and phosphate may be added to alternate infusion bottles to avoid precipitation. In patients with hyperchloremic or other metabolic acidosis, sodium and potassium may be added as acetate or lactate salts to provide bicarbonate precursor. The electrolyte content of NephrAmine® must be considered when calculating daily electrolyte intake. Serum electrolytes, including magnesium and phosphorus, should be monitored frequently.
If a patient's nutritional intake is primarily parenteral, vitamins, especially the water soluble vitamins, should also be provided.
Hypertonic mixtures of essential amino acids and dextrose may be safely administered by continuous infusion through a central venous catheter with the tip located in the superior vena cava. Initial infusion rates should be slow, generally 20–30 mL/hour. Increases by increments of 10 mL/hour each 24 hours are recommended to a maximum of 60–100 mL/hour. If administration rate should fall behind schedule, no attempt to "catch up" to planned intake should be made.
Administration rate is governed by the patient's nitrogen, fluid, and glucose tolerance. Uremic patients are frequently glucose intolerant, especially in association with peritoneal dialysis, and may require the administration of exogenous insulin to prevent hyperglycemia. Blood glucose levels must be determined frequently. To prevent rebound hypoglycemia, a solution containing 5% dextrose should be administered when hypertonic dextrose infusions are abruptly discontinued.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Care must be taken to avoid incompatible admixtures. Consult with pharmacist.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
Directions for Use of B. Braun Glass Containers with Solid Stoppers
Designed for use with a vented set. Use 18 to 22 gauge needle size for admixing or withdrawing solutions from the glass bottle.
- Before use, perform the following checks:
Inspect each container. Read the label. Ensure solution is the one ordered and is within the expiration date.
Invert container and carefully inspect the solution in good light for cloudiness, haze, or particulate matter; check the bottle for cracks or other damage. In checking for cracks, do not be confused by normal surface marks and seams on the bottom and sides of the bottle. These are not flaws. Look for bright reflections that have depth and penetrate into the wall of the bottle. Reject any such bottle. - To remove the outer closure, lift the tear tab and pull up, over, and down until it is below the stopper (see Figure 1). Use a circular pulling motion on the tab until it breaks away.
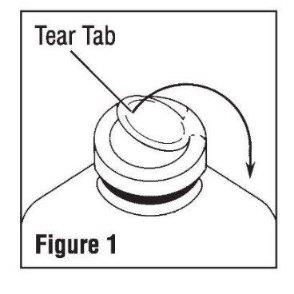
- Grasp and remove the metal disk, exercising caution not to touch the exposed sterile stopper surface.
Warning: Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store. - When adding medication to the container prior to administration, swab the target area of the rubber stopper, inject medication and mix thoroughly by gentle agitation.
- Refer to Directions for Use of the set being used. Insert the set spike into the bottle through the target area of the rubber stopper. Allow the fluid to flow and remove air from the tubing before administration begins. Hang the container.
- After admixture and during administration, re-inspect the solution frequently. If any evidence of solution contamination or instability is found or if the patient exhibits any signs of fever, chills or other reactions not readily explainable, discontinue administration immediately and notify the physician.
- Spiking, additions, or transfers should be made immediately after exposing the sterile stopper surface. Check for vacuum at first puncture of stopper. Admixture by needle or syringe should be made through the target area of the rubber stopper; contents should be drawn by vacuum into the bottle. Admixture by spiked vial should be through the target area of the rubber stopper (See Figure 2). If contents of initial addition are not drawn into the bottle, vacuum is not present and the unit should be discarded. Each addition/transfer will reduce the vacuum remaining in the bottle.
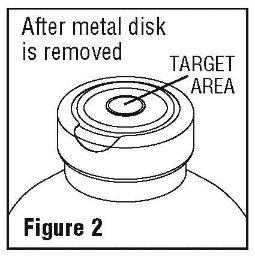
- If the first puncture of the stopper is the administration set spike, insert the spike fully into the target area of the rubber stopper and promptly invert the bottle. Verify vacuum by observing rising air bubbles. Do not use the bottle if vacuum is not present.
- If admixture or set insertion is not performed immediately following removal of protective metal disk, swab stopper surface.
- Before use, perform the following checks:
- SPL UNCLASSIFIED SECTION
-
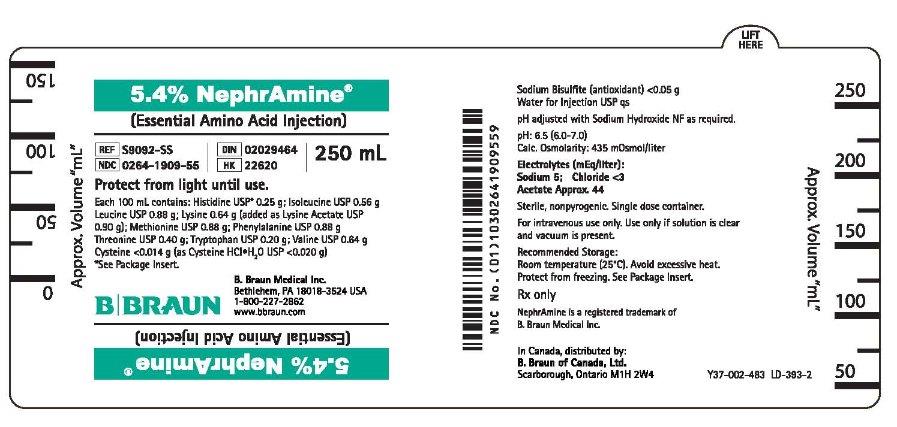
PRINCIPAL DISPLAY PANEL - 250 mL Container Label
5.4% NephrAmine®
(Essential Amino Acid Injection)REF S9092-SS
NDC: 0264-1909-55
DIN 02029464
HK 22620
250 mLProtect from light until use.
Each 100 mL contains: Histidine USP* 0.25 g; Isoleucine USP 0.56 g
Leucine USP 0.88 g; Lysine 0.64 g (added as Lysine Acetate USP
0.90 g); Methionine USP 0.88 g; Phenylalanine USP 0.88 g
Threonine USP 0.40 g; Tryptophan USP 0.20 g; Valine USP 0.64 g
Cysteine <0.014 g (as Cysteine HClH2O USP <0.020 g)*See Package Insert.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
www.bbraun.comSodium Bisulfite (antioxidant) <0.05 g
Water for Injection USP qspH adjusted with Sodium Hydroxide NF as required.
pH: 6.5 (6.0-7.0)
Calc. Osmolarity: 435 mOsmol/literElectrolytes (mEq/liter):
Sodium 5; Chloride <3
Acetate Approx. 44Sterile, nonpyrogenic. Single dose container.
For intravenous use only. Use only if solution is clear
and vacuum is present.Recommended Storage:
Room temperature (25°C). Avoid excessive heat.
Protect from freezing. See Package Insert.Rx only
NephrAmine is a registered trademark
of B. Braun Medical Inc.In Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4Y37-002-483 LD-393-2
-
PRINCIPAL DISPLAY PANEL - 250 mL Container Carton
5.4% NephrAmine®
(Essential Amino Acid Injection)
REF S9092-SS
NDC: 0264-1909-55
DIN 02029464
HK 22620
250 mLProtect from light until use.
Each 100 mL contains:
Histidine USP* 0.25 g; Isoleucine USP 0.56 g
Leucine USP 0.88 g; Lysine 0.64 g (added as Lysine
Acetate USP 0.90 g); Methionine USP 0.88 g
Phenylalanine USP 0.88 g; Threonine USP 0.40 g
Tryptophan USP 0.20 g; Valine USP 0.64 g; Cysteine
<0.014 g (as Cysteine HClH2O USP <0.020 g)Sodium Bisulfite (antioxidant) <0.05 g
Water for Injection USP qspH adjusted with Sodium Hydroxide NF as required.
pH: 6.5 (6.0-7.0)
Calculated Osmolarity: 435 mOsmol/literElectrolytes (mEq/liter):
Sodium 5; Chloride <3; Acetate Approx. 44
*See Package Insert.See adjacent panel for further product information.
Sterile, nonpyrogenic. Single dose container.
For intravenous use only. Use only if solution is clear
and vacuum is present.Recommended Storage:
Room temperature (25°C). Avoid excessive heat.
Protect from freezing. See Package Insert.Rx only
NephrAmine is a registered trademark of B. Braun
Medical Inc.B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
www.bbraun.comIn Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4X12-002-475 LD-199-3

-
INGREDIENTS AND APPEARANCE
NEPHRAMINE
histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine, cysteine, and sodium bisulfite injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-1909 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HISTIDINE (UNII: 4QD397987E) (HISTIDINE - UNII:4QD397987E) HISTIDINE 0.25 g in 100 mL ISOLEUCINE (UNII: 04Y7590D77) (ISOLEUCINE - UNII:04Y7590D77) ISOLEUCINE 0.56 g in 100 mL LEUCINE (UNII: GMW67QNF9C) (LEUCINE - UNII:GMW67QNF9C) LEUCINE 0.88 g in 100 mL LYSINE ACETATE (UNII: TTL6G7LIWZ) (LYSINE - UNII:K3Z4F929H6) LYSINE 0.64 g in 100 mL METHIONINE (UNII: AE28F7PNPL) (METHIONINE - UNII:AE28F7PNPL) METHIONINE 0.88 g in 100 mL PHENYLALANINE (UNII: 47E5O17Y3R) (PHENYLALANINE - UNII:47E5O17Y3R) PHENYLALANINE 0.88 g in 100 mL THREONINE (UNII: 2ZD004190S) (THREONINE - UNII:2ZD004190S) THREONINE 0.4 g in 100 mL TRYPTOPHAN (UNII: 8DUH1N11BX) (TRYPTOPHAN - UNII:8DUH1N11BX) TRYPTOPHAN 0.2 g in 100 mL VALINE (UNII: HG18B9YRS7) (VALINE - UNII:HG18B9YRS7) VALINE 0.64 g in 100 mL CYSTEINE HYDROCHLORIDE (UNII: ZT934N0X4W) (CYSTEINE - UNII:K848JZ4886) CYSTEINE 0.014 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM BISULFITE (UNII: TZX5469Z6I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-1909-55 12 in 1 CASE 02/24/1978 1 1 in 1 CARTON 1 250 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017766 02/24/1978 Labeler - B. Braun Medical Inc. (002397347)
Trademark Results [NephrAmine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NEPHRAMINE 73171357 1111189 Live/Registered |
AMERICAN HOSPITAL SUPPLY CORPORATION 1978-05-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.