Spot Check by Skin PS Brands / Taiki Spot Check
Spot Check by
Drug Labeling and Warnings
Spot Check by is a Otc medication manufactured, distributed, or labeled by Skin PS Brands, Taiki. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SPOT CHECK- acne patches with salicylic acid patch
Skin PS Brands
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
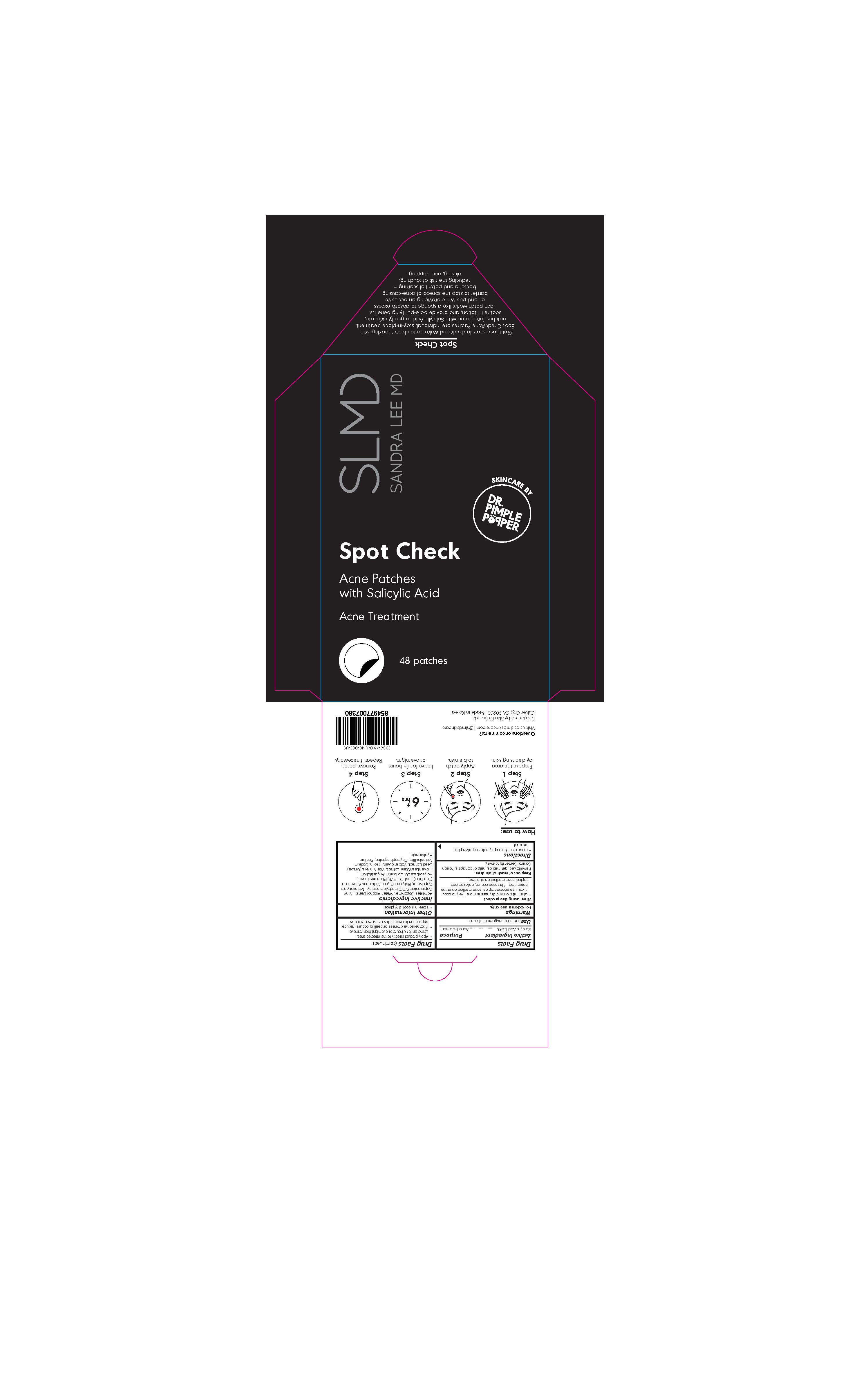
Spot Check
When using this product
- Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Clean skin thoroughly before applying this product
- Apply product directly to the affected area.
- Leave on for 6 hours or overnight then remove.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive Ingredients
Acrylates Copolymer, Water, Alcohol Denat., Vinyl Caprolactam/VP/Dimethylaminoethyl, Methacrylate Copolymer, Butylene Glycol, Melaleuca Alternifolia (Tea Tree) Leaf Oil, PVP, Phenoxyethanol, Polysorbate 80, Epilobium Angustifolium Flower/Leaf/Stem Extract, Vitis Vinifera (Grape) Seed Extract, Volcanic Ash, Kaolin, Sodium Metabisulfite, Phytosphingosine, Sodium Hyaluronate.
How to use:
Step 1
Prepare the area by cleansing Skin
Step 2
Apply patch to blemish
Step 3
Leave for 6+ hours or overnight
Step 4
Remove patch. Repeat if necessary.
Questions or comments?
Visit us at slmdskincare.com | @slmdskincare
Distributed by Skin PS Brands
Culver City, CA 90232 | Made in Korea
Spot Check
Get those spots in check and wake up to clearer-looking skin. Spot Check Acne Patches are individual, stay-in-place treatment patches formulated with Salicylic Acid to gently exfoliate, soothe irritation, and provide pore-purifying benefits. Each patch works like a sponge to absorb excess oil and pus, while providing an occlusive barrier to stop the spread of acne-causing bacteria and potential scarring - reducing the risk of touching, picking, and popping.
| SPOT CHECK
acne patches with salicylic acid patch |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Skin PS Brands (081085221) |
| Registrant - Skin PS Brands (081085221) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Taiki | 688783559 | manufacture(73318-1034) | |
Trademark Results [Spot Check]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 Spot Check Sachet (Packette)
Spot Check Sachet (Packette)