Nystatin by TriRx Huntsville Pharmaceutical Services, LLC
Nystatin by
Drug Labeling and Warnings
Nystatin by is a Prescription medication manufactured, distributed, or labeled by TriRx Huntsville Pharmaceutical Services, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
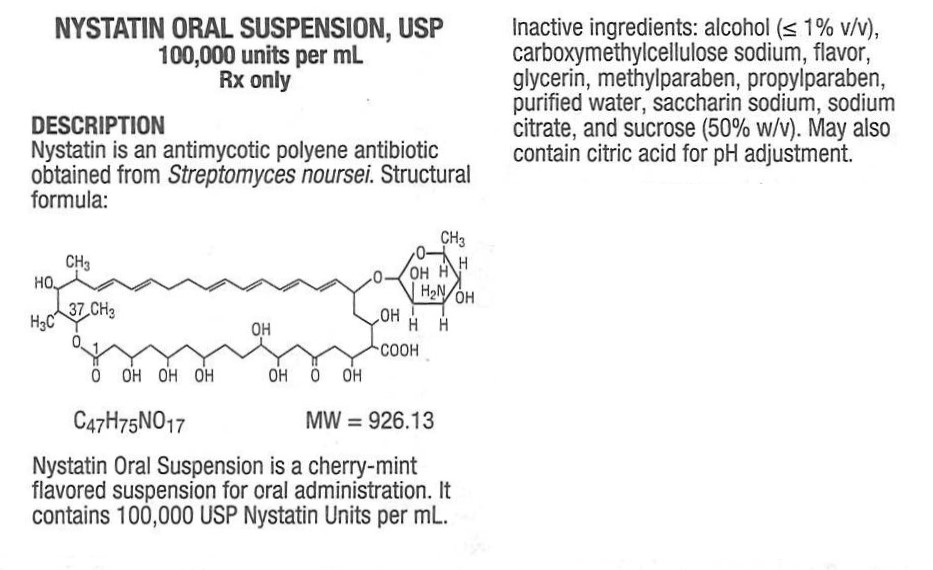
NYSTATIN- nystatin oral suspension suspension
TriRx Huntsville Pharmaceutical Services, LLC
----------
| NYSTATIN
nystatin oral suspension suspension |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - TriRx Huntsville Pharmaceutical Services, LLC (117090286) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TriRx Huntsville Pharmaceutical Services, LLC | 117090286 | manufacture(80432-003) | |
Revised: 8/2024
Document Id: 1f7d60cd-389e-f973-e063-6294a90a7376
Set id: b95ce95e-ea57-99d5-e053-2a95a90ab217
Version: 4
Effective Time: 20240812
TriRx
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.