PPE Wipes by ASC Marketing LTD. PPE Wipes
PPE Wipes by
Drug Labeling and Warnings
PPE Wipes by is a Otc medication manufactured, distributed, or labeled by ASC Marketing LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
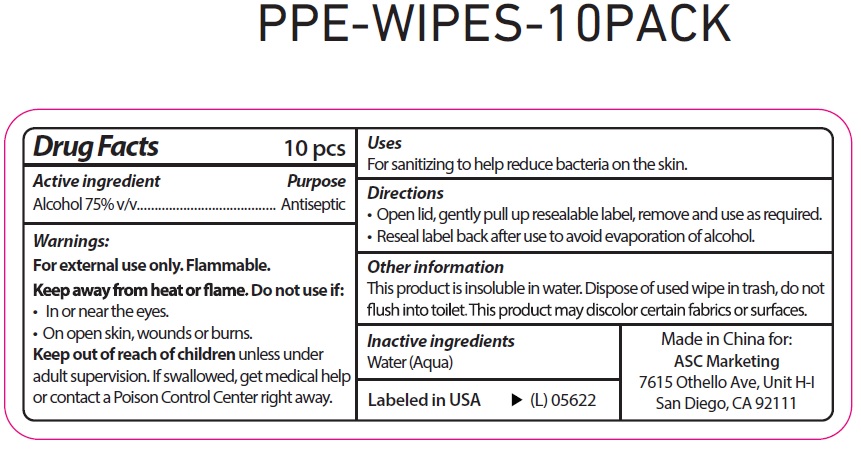
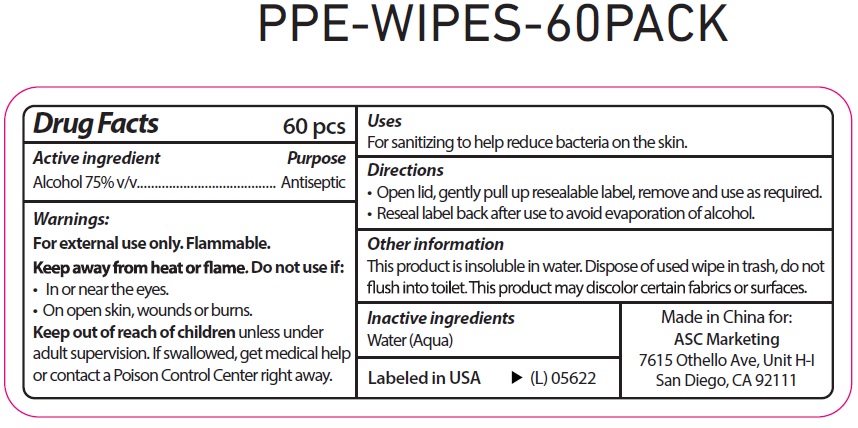
PPE WIPES- alcohol cloth
ASC Marketing LTD.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
PPE Wipes
Directions
Open lid, gently pull up resealable label, remove and use as required.
Reseal label back after use to avoid evaporation of alcohol.
| PPE WIPES
alcohol cloth |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - ASC Marketing LTD. (117025198) |
Revised: 4/2022
Document Id: dbeae7b1-d708-3e64-e053-2995a90af46a
Set id: b97ffd64-4967-244a-e053-2a95a90ad2bc
Version: 2
Effective Time: 20220405
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.