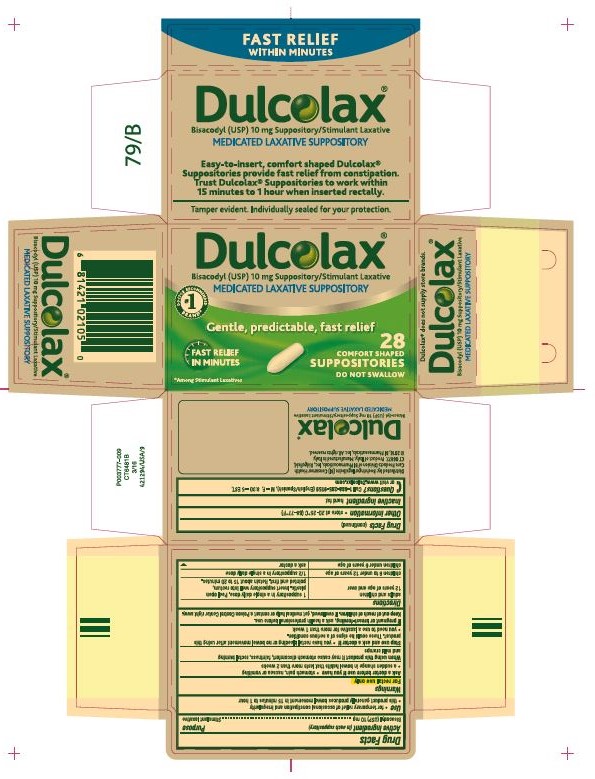
DULCOLAX- bisacodyl suppository
Dulcolax by
Drug Labeling and Warnings
Dulcolax by is a Otc medication manufactured, distributed, or labeled by Boehringer Ingelheim Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
-
WARNINGS
Ask a doctor before use if you have
- stomach pain, nausea or vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
When using this product it may cause stomach discomfort, faintness, rectal burning and mild cramps
-
DOSAGE & ADMINISTRATION
adults and children 12 years of age and over 1 suppository in a single daily dose. Peel open plastic. Insert suppository well into rectum, pointed end first. Retain about 15 to 20 minutes. children 6 to under 12 years of age 1/2 suppository in a single daily dose children under 6 years of age ask a doctor - STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Questions? call 1-888-285-9159 (English/Spanish), M – F, 8:30 – 5 EST, or visit www.Dulcolax.com
Distributed by: Boehringer Ingelheim (BI) Consumer Health Care Products Division of BI Pharmaceuticals, Inc., Ridgefield, CT 06877. Product of Italy. Manufactured in Italy. © 2016, BI Pharmaceuticals, Inc. All rights reserved.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DULCOLAX
bisacodyl suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0597-0052 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISACODYL (UNII: 10X0709Y6I) (DEACETYLBISACODYL - UNII:R09078E41Y) BISACODYL 10 mg Inactive Ingredients Ingredient Name Strength FAT, HARD (UNII: 8334LX7S21) Product Characteristics Color WHITE Score Shape BULLET Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0597-0052-16 4 in 1 CARTON 04/01/2002 1 4 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 0597-0052-28 7 in 1 CARTON 04/01/2002 2 4 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 0597-0052-34 1 in 1 CARTON 04/01/2002 3 4 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC: 0597-0052-38 2 in 1 CARTON 04/01/2002 4 4 in 1 BLISTER PACK; Type 0: Not a Combination Product 5 NDC: 0597-0052-44 1 in 1 CARTON 04/01/2002 5 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 04/01/2002 Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944)
Trademark Results [Dulcolax]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DULCOLAX 79043985 3505530 Live/Registered |
Sanofi-Aventis Deutschland GmbH 2007-08-16 |
 DULCOLAX 78157653 2861373 Dead/Cancelled |
Boehringer Ingelheim International GmbH 2002-08-26 |
 DULCOLAX 72042116 0671422 Live/Registered |
DR, KARL THOMAE GMBH 1957-12-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.