AIMOVIG- erenumab-aooe injection AIMOVIG- erenumab-aooe injection, solution
AIMOVIG by
Drug Labeling and Warnings
AIMOVIG by is a Prescription medication manufactured, distributed, or labeled by Amgen Inc, Amgen Manufacturing Ltd, Amgen Technology (ireland) Unlimited Company, Amgen, Inc, BioReliance Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AIMOVIG safely and effectively. See full prescribing information for AIMOVIG.
AIMOVIG® (erenumab-aooe) injection, for subcutaneous use
Initial U.S. Approval: 2018
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
AIMOVIG is a calcitonin gene-related peptide receptor antagonist indicated for the preventive treatment of migraine in adults (1).
DOSAGE AND ADMINISTRATION
- For subcutaneous use only (2.1, 2.2)
- Recommended dosage is 70 mg once monthly; some patients may benefit from a dosage of 140 mg once monthly (2.1)
- The needle shield within the white or orange cap of the prefilled autoinjector and the gray needle cap of the prefilled syringe contain dry natural rubber (a derivative of latex), which may cause allergic reactions in individuals sensitive to latex (2.2)
- Administer in the abdomen, thigh, or upper arm subcutaneously (2.2)
- See Dosage and Administration for important administration instructions (2.2)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
AIMOVIG is contraindicated in patients with serious hypersensitivity to erenumab-aooe or to any of the excipients. (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: If a serious hypersensitivity reaction occurs, discontinue administration of AIMOVIG and initiate appropriate therapy. Hypersensitivity reactions can occur within hours to more than one week after administration. (5.1)
- Constipation with Serious Complications: Serious complications of constipation may occur. (5.2)
ADVERSE REACTIONS
The most common adverse reactions in AIMOVIG clinical studies (occurring in at least 3% of treated patients and more often than placebo) are injection site reactions and constipation. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amgen Medical Information at 1-800-77-AMGEN (1-800-772-6436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2019
- For subcutaneous use only (2.1, 2.2)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Constipation with Serious Complications
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2
DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
The recommended dosage of AIMOVIG is 70 mg injected subcutaneously once monthly. Some patients may benefit from a dosage of 140 mg injected subcutaneously once monthly.
If a dose of AIMOVIG is missed, administer as soon as possible. Thereafter, AIMOVIG can be scheduled monthly from the date of the last dose.
2.2 Important Administration Instructions
AIMOVIG is for subcutaneous use only.
The needle shield within the white or orange cap of the AIMOVIG prefilled autoinjector and gray needle cap of the AIMOVIG prefilled syringe contain dry natural rubber (a derivative of latex), which may cause allergic reactions in individuals sensitive to latex.
AIMOVIG is intended for patient self-administration. Prior to use, provide proper training to patients and/or caregivers on how to prepare and administer AIMOVIG using the single-dose prefilled autoinjector or single-dose prefilled syringe, including aseptic technique [see Instructions for Use]:
- Prior to subcutaneous administration, allow AIMOVIG to sit at room temperature for at least 30 minutes protected from direct sunlight [see How Supplied/Storage and Handling (16.2)]. Do not warm by using a heat source such as hot water or a microwave.
- Do not shake the product.
- Inspect visually for particulate matter and discoloration prior to administration [see Dosage Forms and Strengths (3)]. Do not use if the solution is cloudy or discolored or contains flakes or particles.
- Administer AIMOVIG in the abdomen, thigh, or upper arm subcutaneously. Do not inject into areas where the skin is tender, bruised, red, or hard.
- Both prefilled autoinjector and prefilled syringe are single-dose and deliver the entire contents.
- Prior to subcutaneous administration, allow AIMOVIG to sit at room temperature for at least 30 minutes protected from direct sunlight [see How Supplied/Storage and Handling (16.2)]. Do not warm by using a heat source such as hot water or a microwave.
-
3
DOSAGE FORMS AND STRENGTHS
AIMOVIG is a sterile, clear to opalescent, colorless to light yellow solution available as follows:
- Injection: 70 mg/mL in a single-dose prefilled SureClick autoinjector
- Injection: 140 mg/mL in a single-dose prefilled SureClick autoinjector
- Injection: 70 mg/mL in a single-dose prefilled syringe
- Injection: 140 mg/mL in a single-dose prefilled syringe
- Injection: 70 mg/mL in a single-dose prefilled SureClick autoinjector
-
4
CONTRAINDICATIONS
AIMOVIG is contraindicated in patients with serious hypersensitivity to erenumab-aooe or to any of the excipients. Reactions have included anaphylaxis and angioedema [see Warnings and Precautions (5.1)].
-
5
WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including rash, angioedema, and anaphylaxis, have been reported with AIMOVIG in postmarketing experience. Most hypersensitivity reactions were not serious and occurred within hours of administration, although some occurred more than one week after administration. If a serious or severe hypersensitivity reaction occurs, discontinue administration of AIMOVIG and initiate appropriate therapy [see Contraindications (4), and Patient Counseling Information (17)].
5.2 Constipation with Serious Complications
Constipation with serious complications has been reported following the use of AIMOVIG in the postmarketing setting. There were cases that required hospitalization, including cases where surgery was necessary. In a majority of these cases, the onset of constipation was reported after the first dose of AIMOVIG; however, patients have also presented with constipation later on in treatment. AIMOVIG was discontinued in most reported cases of constipation with serious complications. Constipation was one of the most common (up to 3%) adverse reactions reported in clinical studies [see Adverse Reactions (6.1)].
Monitor patients treated with AIMOVIG for severe constipation and manage as clinically appropriate [see Patient Counseling Information (17)]. The concurrent use of medications associated with decreased gastrointestinal motility may increase the risk for more severe constipation and the potential for constipation-related complications.
-
6
ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Constipation with Serious Complications [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of AIMOVIG has been evaluated in 2537 patients with migraine who received at least one dose of AIMOVIG, representing 2310 patient-years of exposure. Of these, 2057 patients were exposed to 70 mg or 140 mg once monthly for at least 6 months, 1198 patients were exposed for at least 12 months, and 287 patients were exposed for at least 18 months.
In placebo-controlled clinical studies (Studies 1, 2, and 3) of 2184 patients, 787 patients received at least one dose of AIMOVIG 70 mg once monthly, 507 patients received at least one dose of AIMOVIG 140 mg once monthly, and 890 patients received placebo during 3 months or 6 months of double-blind treatment [see Clinical Studies (14)]. Approximately 84% were female, 91% were white, and the mean age was 42 years at study entry.
The most common adverse reactions (incidence ≥ 3% and more often than placebo) in the migraine studies were injection site reactions and constipation. Table 1 summarizes the adverse reactions that occurred during the first 3 months in the migraine studies (Studies 1, 2, and 3).
Table 1: Adverse Reactions Occurring with an Incidence of at Least 2% for Either Dose of AIMOVIG and at Least 2% Greater than Placebo During the First 3 Months in Studies 1, 2, and 3 Adverse Reaction AIMOVIG
70 mg Once Monthly
N = 787
%AIMOVIG
140 mg Once Monthly
N = 507
%Placebo
N = 890
%Injection site reactionsa,b 6 5 3 Constipation 1 3 1 Cramps, muscle spasms < 1 2 < 1 a Injection site reactions include multiple adverse reactions related terms, such as injection site pain and injection site erythema.
b The rate of injection site reactions reported in Table 1 is with the prefilled syringe.In Studies 1, 2, and 3, 1.3% of patients treated with AIMOVIG discontinued double-blind treatment because of adverse events. The most frequent injection site reactions were injection site pain, injection site erythema, and injection site pruritus.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation, including neutralizing antibodies, is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to erenumab-aooe in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
The immunogenicity of AIMOVIG has been evaluated using an immunoassay for the detection of binding anti-erenumab-aooe antibodies. For patients whose sera tested positive in the screening immunoassay, an in vitro biological assay was performed to detect neutralizing antibodies.
In controlled studies with AIMOVIG, the incidence of anti-erenumab-aooe antibody development was 6.2% (48/778) in patients receiving AIMOVIG 70 mg once monthly (2 of whom had in vitro neutralizing activity) and 2.6% (13/504) in patients receiving AIMOVIG 140 mg once monthly (none of whom had in vitro neutralizing activity). The neutralizing anti-erenumab-aooe antibody positive rate may be underestimated because of limitations of the assay. Although these data do not demonstrate an impact of anti-erenumab-aooe antibody development on the efficacy or safety of AIMOVIG in these patients, the available data are too limited to make definitive conclusions.
6.3 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of AIMOVIG. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and Appendages: Hypersensitivity reactions, including rash, angioedema, and anaphylaxis [see Warnings and Precautions (5.1)].
Gastrointestinal Disorders: Constipation with serious complications [see Warnings and Precautions (5.2)].
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
-
8
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with the use of AIMOVIG in pregnant women. No adverse effects on offspring were observed when pregnant monkeys were administered erenumab-aooe throughout gestation [see Data]. Serum erenumab-aooe exposures in pregnant monkeys were greater than those in humans at clinical doses.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively. The estimated rate of major birth defects (2.2%-2.9%) and miscarriage (17%) among deliveries to women with migraine are similar to rates reported in women without migraine.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Published data have suggested that women with migraine may be at increased risk of preeclampsia during pregnancy.
Data
Animal Data
In a study in which female monkeys were administered erenumab-aooe (0 or 50 mg/kg) twice weekly by subcutaneous injection throughout pregnancy (gestation day 20-22 to parturition), no adverse effects on offspring were observed. Serum erenumab-aooe exposures (AUC) in pregnant monkeys were approximately 20 times that in humans at a dose of 140 mg once monthly.
8.2 Lactation
Risk Summary
There are no data on the presence of erenumab-aooe in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for AIMOVIG and any potential adverse effects on the breastfed infant from AIMOVIG or from the underlying maternal condition.
8.5 Geriatric Use
Clinical studies of AIMOVIG did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
11
DESCRIPTION
Erenumab-aooe is a human immunoglobulin G2 (IgG2) monoclonal antibody that has high affinity binding to the calcitonin gene-related peptide receptor. Erenumab-aooe is produced using recombinant DNA technology in Chinese hamster ovary (CHO) cells. It is composed of 2 heavy chains, each containing 456 amino acids, and 2 light chains of the lambda subclass, each containing 216 amino acids, with an approximate molecular weight of 150 kDa.
AIMOVIG (erenumab-aooe) injection is supplied as a sterile, preservative-free, clear to opalescent, colorless to light yellow solution for subcutaneous administration. Each 1 mL 70 mg single-dose prefilled autoinjector and 70 mg single-dose prefilled glass syringe contains 70 mg erenumab-aooe, acetate (1.5 mg), polysorbate 80 (0.10 mg), and sucrose (73 mg). Each 1 mL 140 mg single-dose prefilled autoinjector and 140 mg single-dose prefilled glass syringe contains 140 mg erenumab-aooe, acetate (2.0 mg), polysorbate 80 (0.10 mg), and sucrose (65 mg). Enclosed within the autoinjector is a single-dose, prefilled glass syringe. The solution of AIMOVIG has a pH of 5.2.
-
12
CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Erenumab-aooe is a human monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) receptor and antagonizes CGRP receptor function.
12.2 Pharmacodynamics
In a randomized, double-blind, placebo-controlled study in healthy volunteers, concomitant administration of erenumab-aooe (140 mg intravenous, single-dose) with sumatriptan (12 mg subcutaneous, given as two 6 mg doses separated by one hour) had no effect on resting blood pressure compared with sumatriptan alone. AIMOVIG is for subcutaneous use only.
12.3 Pharmacokinetics
Erenumab-aooe exhibits non-linear kinetics as a result of binding to the CGRP receptor. The Cmax mean and AUClast mean following subcutaneous administration of a 70 mg once monthly and a 140 mg once monthly dose in healthy volunteers or migraine patients are included in Table 2.
Less than 2-fold accumulation was observed in trough serum concentrations (Cmin) for episodic and chronic migraine patients following subcutaneous administration of 70 mg once monthly and 140 mg once monthly doses (see Table 2). Serum trough concentrations approached steady state by 3 months of dosing. The effective half-life of erenumab-aooe is 28 days.
Table 2: Pharmacokinetic Parameters of AIMOVIG AIMOVIG 70 mg
Subcutaneously Once MonthlyAIMOVIG 140 mg
Subcutaneously Once MonthlyCmax mean (SD)a,b 6.1 (2.1) mcg/mL 15.8 (4.8) mcg/mL AUClast mean (SD)a,b 159 (58) day*mcg/mL 505 (139) day*mcg/mL Cmin (SD) Episodic migraine 5.7 (3.1) mcg/mL 12.8 (6.5) mcg/mL Chronic migraine 6.2 (2.9) mcg/mL 14.9 (6.5) mcg/mL a SD = standard deviation
b from a single-dose studyAbsorption
Following a single subcutaneous dose of 70 mg or 140 mg erenumab-aooe administered to healthy adults, median peak serum concentrations were attained in approximately 6 days, and estimated absolute bioavailability was 82%.
Distribution
Following a single 140 mg intravenous dose, the mean (SD) volume of distribution during the terminal phase (Vz) was estimated to be 3.86 (0.77) L.
Metabolism and Excretion
Two elimination phases were observed for erenumab-aooe. At low concentrations, the elimination is predominantly through saturable binding to target (CGRP receptor), while at higher concentrations the elimination of erenumab-aooe is largely through a non-specific, non-saturable proteolytic pathway.
Specific Populations
The pharmacokinetics of erenumab-aooe were not affected by age, gender, race, or subtypes of migraine spectrum (episodic or chronic migraine) based on population pharmacokinetics analysis.
Patients with Renal or Hepatic Impairment
Population pharmacokinetic analysis of integrated data from the AIMOVIG clinical studies did not reveal a difference in the pharmacokinetics of erenumab-aooe in patients with mild or moderate renal impairment relative to those with normal renal function. Patients with severe renal impairment (eGFR < 30 mL/min/1.73 m2) have not been studied. No dedicated clinical studies were conducted to evaluate the effect of hepatic impairment or renal impairment on the pharmacokinetics of erenumab-aooe. Renal or hepatic impairment is not expected to affect pharmacokinetics of erenumab-aooe.
Drug Interaction Studies
P450 Enzymes
Erenumab-aooe is not metabolized by cytochrome P450 enzymes; therefore, interactions with concomitant medications that are substrates, inducers, or inhibitors of cytochrome P450 enzymes are unlikely.
Oral Contraceptives
In an open-label drug interaction study in healthy female volunteers, erenumab-aooe (140 mg subcutaneous, single-dose) did not affect the pharmacokinetics of a combined oral contraceptive containing ethinyl estradiol and norgestimate.
Sumatriptan
In a study in healthy volunteers, concomitant administration of erenumab-aooe with sumatriptan had no effect on the pharmacokinetics of sumatriptan [see Clinical Pharmacology (12.2)].
-
13
NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
The carcinogenic potential of erenumab-aooe has not been assessed.
Mutagenesis
Genetic toxicology studies of erenumab-aooe have not been conducted.
Impairment of Fertility
Mating studies have not been conducted on erenumab-aooe. No histopathological changes in male or female reproductive organs were observed in monkeys administered erenumab-aooe (0, 25, or 150 mg/kg) by subcutaneous injection twice weekly for up to 6 months. Serum erenumab-aooe exposures (AUC) at the higher dose tested were more than 100 times that in humans at a dose of 140 mg once monthly.
-
14
CLINICAL STUDIES
The efficacy of AIMOVIG was evaluated as a preventive treatment of episodic or chronic migraine in three randomized, double-blind, placebo-controlled studies: two studies in patients with episodic migraine (4 to 14 migraine days per month) (Study 1 and Study 2) and one study in patients with chronic migraine (≥ 15 headache days per month with ≥ 8 migraine days per month) (Study 3). The studies enrolled patients with a history of migraine, with or without aura, according to the International Classification of Headache Disorders (ICHD-III) diagnostic criteria.
Episodic Migraine
Study 1 (NCT 02456740) was a randomized, multi-center, 6-month, placebo-controlled, double-blind study evaluating AIMOVIG for the preventive treatment of episodic migraine. A total of 955 patients with a history of episodic migraine were randomized to receive either AIMOVIG 70 mg (N = 317), AIMOVIG 140 mg (N = 319), or placebo (N = 319) by subcutaneous injection once monthly (QM) for 6 months. Patients were allowed to use acute headache treatments including migraine-specific medications (i.e., triptans, ergotamine derivatives) and NSAIDs during the study.
The study excluded patients with medication overuse headache as well as patients with myocardial infarction, stroke, transient ischemic attacks, unstable angina, coronary artery bypass surgery, or other revascularization procedures within 12 months prior to screening.
The primary efficacy endpoint was the change from baseline in mean monthly migraine days over months 4 to 6. Secondary endpoints included the achievement of a ≥ 50% reduction from baseline in mean monthly migraine days over months 4 to 6 (“≥ 50% MMD responders”), the change from baseline in mean monthly acute migraine-specific medication days over months 4 to 6, and the change from baseline in mean Migraine Physical Function Impact Diary (MPFID) over months 4 to 6. The MPFID measures the impact of migraine on everyday activities (EA) and physical impairment (PI) using an electronic diary administered daily. Monthly MPFID scores are averaged over 28 days, including days with and without migraine; scores are scaled from 0 to 100. Higher scores indicate worse impact on EA and PI. Reductions from baseline in MPFID scores indicate improvement.
A total of 858 (90%) patients completed the 6-month double-blind study. Patients had a median age of 42 years (range: 18 to 65 years), 85% were female, and 89% were white. Three percent of patients were taking concomitant preventive treatments for migraine. The mean migraine frequency at baseline was approximately 8 migraine days per month and was similar across treatment groups.
AIMOVIG treatment demonstrated statistically significant improvements for key efficacy endpoints compared to placebo, as summarized in Table 3.
Table 3: Efficacy Endpoints Over Months 4 to 6 in Study 1 AIMOVIG
70 mg Once Monthly
N = 312AIMOVIG
140 mg Once Monthly
N = 318Placebo
N = 316Monthly Migraine Days (MMD) Change from baseline -3.2 -3.7 -1.8 Difference from placebo -1.4 -1.9 p-value < 0.001 < 0.001 ≥ 50% MMD responders % Responders 43.3% 50.0% 26.6% Difference from placebo 16.7% 23.4% Odds ratio relative to placebo 2.1 2.8 p-value < 0.001 < 0.001 Monthly acute migraine-specific medication days Change from baseline -1.1 -1.6 -0.2 Difference from placebo -0.9 -1.4 p-value < 0.001 < 0.001 Figure 1: Change from Baseline in Monthly Migraine Days in Study 1a 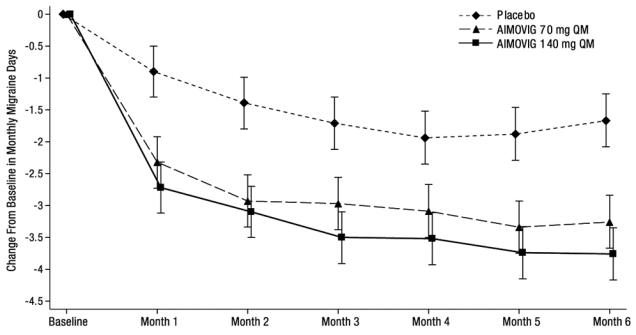
a Least-square means and 95% confidence intervals are presented. Figure 2 shows the distribution of change from baseline in mean monthly migraine days over months 4 to 6 in bins of 2 days by treatment group. A treatment benefit over placebo for both doses of AIMOVIG is seen across a range of changes from baseline in monthly migraine days.
Figure 2: Distribution of Change from Baseline in Mean Monthly Migraine Days Over Months 4 to 6 by Treatment Group in Study 1
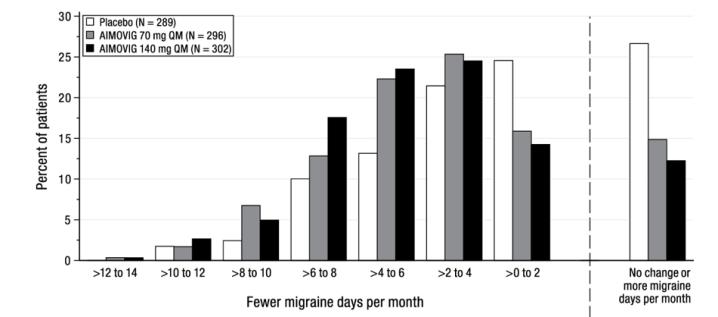
Figure excludes patients with missing data. Compared to placebo, patients treated with AIMOVIG 70 mg once monthly and 140 mg once monthly showed greater reductions from baseline in mean monthly MPFID everyday activity scores averaged over months 4 to 6 [difference from placebo: -2.2 for AIMOVIG 70 mg and -2.6 for AIMOVIG 140 mg; p-value < 0.001 for both], and in mean monthly MPFID physical impairment scores averaged over months 4 to 6 [difference from placebo: -1.9 for AIMOVIG 70 mg and -2.4 for AIMOVIG 140 mg; p-value < 0.001 for both].
Study 2 (NCT 02483585) was a randomized, multi-center, 3-month, placebo-controlled, double-blind study evaluating AIMOVIG for the preventive treatment of episodic migraine. A total of 577 patients with a history of episodic migraine were randomized to receive either AIMOVIG 70 mg (N = 286) or placebo (N = 291) by subcutaneous injection once monthly for 3 months. Patients were allowed to use acute headache treatments including migraine-specific medications (i.e., triptans, ergotamine derivatives) and NSAIDs during the study.
The study excluded patients with medication overuse headache as well as patients with myocardial infarction, stroke, transient ischemic attacks, unstable angina, coronary artery bypass surgery, or other revascularization procedures within 12 months prior to screening.
The primary efficacy endpoint was the change from baseline in monthly migraine days at month 3. Secondary endpoints included the achievement of a ≥ 50% reduction from baseline in monthly migraine days (“≥ 50% MMD responders”), the change from baseline in monthly acute migraine-specific medication days at month 3, and the proportion of patients with at least a 5-point score reduction from baseline in MPFID at month 3.
A total of 546 (95%) patients completed the 3-month double-blind study. Patients had a median age of 43 years (range: 18 to 65 years), 85% were female, and 90% were white. Six to seven percent of patients were taking concomitant preventive migraine treatment. The mean migraine frequency at baseline was approximately 8 migraine days per month and was similar between treatment groups.
AIMOVIG treatment demonstrated statistically significant improvements for key efficacy endpoints compared to placebo, as summarized in Table 4.
Table 4: Efficacy Endpoints at Month 3 for Study 2 AIMOVIG
70 mg Once Monthly
N = 282Placebo
N = 288Monthly Migraine Days (MMD) Change from baseline -2.9 -1.8 Difference from placebo -1.0 p-value < 0.001 ≥ 50% MMD responders % Responders 39.7% 29.5% Difference from placebo 10.2% Odds ratio relative to placebo 1.6 p-value 0.010 Monthly acute migraine-specific medication days Change from baseline -1.2 -0.6 Difference from placebo -0.6 p-value 0.002 Figure 3: Change from Baseline in Monthly Migraine Days in Study 2a
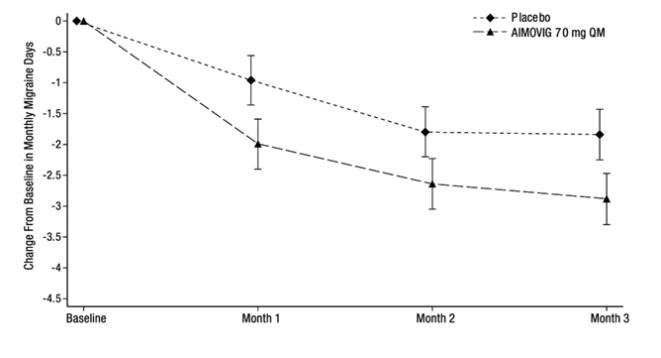
a Least-square means and 95% confidence intervals are presented. Figure 4 shows the distribution of change from baseline in monthly migraine days at month 3 in bins of 2 days by treatment group. A treatment benefit over placebo for AIMOVIG is seen across a range of changes from baseline in monthly migraine days.
Figure 4: Distribution of Change from Baseline in Monthly Migraine Days at Month 3 by Treatment Group in Study 2
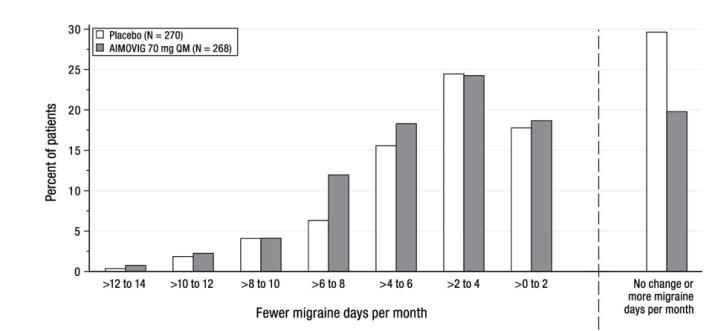
Figure excludes patients with missing data. The pre-specified analysis for the MPFID was based on at least a 5-point reduction within-patient responder definition. AIMOVIG 70 mg once monthly was not significantly better than placebo for the proportion of responders for everyday activity [difference from placebo: 4.7%; odds ratio = 1.2; p-value = 0.26] and physical impairment [difference from placebo: 5.9%; odds ratio = 1.3; p-value = 0.13]. In an exploratory analysis of the change from baseline in the mean MPFID scores at month 3, patients treated with AIMOVIG 70 mg, as compared to placebo, showed nominally greater reductions of physical impairment scores [difference from placebo: -1.3; p-value = 0.021], but not of everyday activities scores [difference from placebo: -1.1; p-value = 0.061].
Chronic Migraine
Study 3 (NCT 02066415) was a randomized, multi-center, 3-month, placebo-controlled, double-blind study evaluating AIMOVIG as a preventive treatment of chronic migraine. A total of 667 patients with a history of chronic migraine with or without aura were randomized to receive AIMOVIG 70 mg (N = 191), AIMOVIG 140 mg (N = 190), or placebo (N = 286) by subcutaneous injections once monthly for 3 months. Patients were allowed to use acute headache treatments including migraine-specific medications (i.e., triptans, ergotamine derivatives) and NSAIDs during the study.
The study excluded patients with medication overuse headache caused by opiate overuse and patients with concurrent use of migraine preventive treatments. Patients with myocardial infarction, stroke, transient ischemic attacks, unstable angina, coronary artery bypass surgery, or other revascularization procedures within 12 months prior to screening were also excluded.
The primary efficacy endpoint was the change from baseline in monthly migraine days at month 3. Secondary endpoints included the achievement of a ≥ 50% reduction from baseline in monthly migraine days (“≥ 50% MMD responders”) and change from baseline in monthly acute migraine-specific medication days at month 3.
A total of 631 (95%) patients completed the 3-month double-blind study. Patients had a median age of 43 years (range: 18 to 66 years), 83% were female, and 94% were white. The mean migraine frequency at baseline was approximately 18 migraine days per month and was similar across treatment groups.
AIMOVIG treatment demonstrated statistically significant improvements for key efficacy outcomes compared to placebo, as summarized in Table 5.
Table 5: Efficacy Endpoints at Month 3 in Study 3 AIMOVIG
70 mg Once Monthly
N = 188AIMOVIG
140 mg Once Monthly
N = 187Placebo
N = 281Monthly Migraine Days (MMD) Change from baseline -6.6 -6.6 -4.2 Difference from placebo -2.5 -2.5 p-value < 0.001 < 0.001 ≥ 50% MMD responders % Responders 39.9% 41.2% 23.5% Difference from placebo 16.4% 17.7% Odds ratio relative to placebo 2.2 2.3 p-value < 0.001 < 0.001 Monthly acute migraine-specific medication days Change from baseline -3.5 -4.1 -1.6 Difference from placebo -1.9 -2.6 p-value < 0.001 < 0.001 Figure 5: Change from Baseline in Monthly Migraine Days in Study 3a
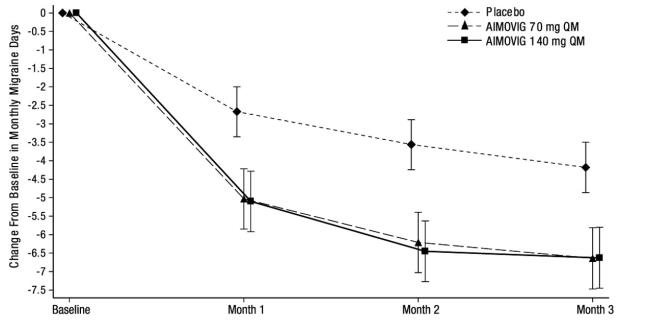
a Least-square means and 95% confidence intervals are presented. Figure 6 shows the distribution of change from baseline in monthly migraine days at month 3 in bins of 3 days by treatment group. A treatment benefit over placebo for both doses of AIMOVIG is seen across a range of changes from baseline in migraine days.
Figure 6: Distribution of Change from Baseline in Monthly Migraine Days at Month 3 by Treatment Group in Study 3 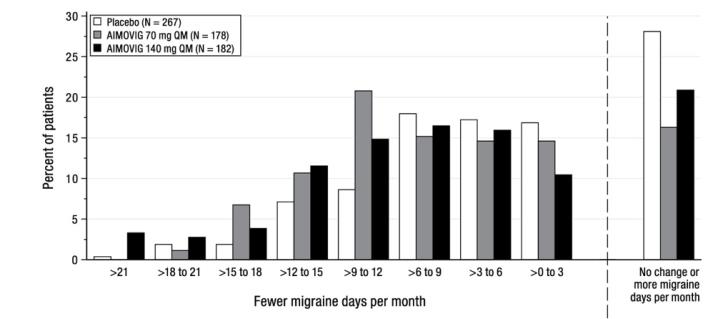
Figure excludes patients with missing data. -
16
HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
AIMOVIG (erenumab-aooe) injection is a sterile, clear to opalescent, colorless to light yellow solution for subcutaneous administration.
The needle shield within the white or orange cap of the AIMOVIG prefilled autoinjector and gray needle cap of the AIMOVIG prefilled syringe contain dry natural rubber (a derivative of latex). Each single-dose prefilled SureClick® autoinjector or single-dose prefilled syringe of AIMOVIG contains a Type 1 glass syringe and stainless steel needle and delivers 1 mL of 70 mg/mL or 140 mg/mL solution.
AIMOVIG is supplied as follows:
SureClick® Autoinjector
- Pack of 1 autoinjector: 70 mg/mL single-dose prefilled autoinjector
NDC: 55513-841-01
- Pack of 1 autoinjector: 140 mg/mL single-dose prefilled autoinjector
NDC: 55513-843-01
Syringe
- Pack of 1 syringe: 70 mg/mL single-dose prefilled syringe
NDC: 55513-840-01
- Pack of 1 syringe: 140 mg/mL single-dose prefilled syringe
NDC: 55513-842-01
16.2 Storage and Handling
- Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light until time of use.
- If removed from the refrigerator, AIMOVIG should be kept at room temperature (up to 25°C [77°F]) in the original carton and must be used within 7 days. Throw away AIMOVIG that has been left at room temperature for more than 7 days.
- Do not freeze.
- Do not shake.
-
17
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Information on Preparation and Administration:
Provide guidance to patients and caregivers on proper subcutaneous administration technique, including aseptic technique, and how to use the single-dose prefilled autoinjector or single-dose prefilled syringe [see Dosage and Administration (2.2)]. Instruct patients and/or caregivers to read and follow the Instructions for Use each time they use AIMOVIG.
Advise latex-sensitive patients that the needle shield within the white or orange cap of the AIMOVIG prefilled autoinjector and gray needle cap of the AIMOVIG prefilled syringe contain dry natural rubber (a derivative of latex) that may cause allergic reactions in individuals sensitive to latex [see Dosage and Administration (2.2)].
Hypersensitivity Reactions:
Advise patients to seek immediate medical attention if they experience any symptoms of serious or severe hypersensitivity reactions [see Warnings and Precautions (5.1)].
Constipation with Serious Complications:
Advise patients that constipation with serious complications can occur with AIMOVIG and that they should contact their healthcare providers if they experience severe constipation [see Warnings and Precautions (5.2)].
For more information, go to www.aimovig.com or call 1-800-77-AMGEN (1-800-772-6436).
AIMOVIG® (erenumab-aooe)
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, CA 91320-1799 U.S.A.
U.S. License No. 1080Marketed by:
Amgen Inc. (Thousand Oaks, CA 91320), and
Novartis Pharmaceuticals Corporation (East Hanover, NJ 07936)Patent: http://pat.amgen.com/aimovig/
© 2018-2019 Amgen Inc. All rights reserved.
[part number] V3 -
PATIENT PACKAGE INSERT
Patient Information
AIMOVIG® (AIM-oh-vig) (erenumab-aooe)
injection, for subcutaneous useWhat is AIMOVIG?
AIMOVIG is a prescription medicine used for the preventive treatment of migraine in adults.
It is not known if AIMOVIG is safe and effective in children under 18 years of age.Who should not use AIMOVIG?
Do not use AIMOVIG if you are allergic to erenumab-aooe or any of the ingredients in AIMOVIG. See the end of this Patient information for a complete list of ingredients in AIMOVIG.Before you start using AIMOVIG, tell your healthcare provider about all your medical conditions, including if you are:
-
Allergic to rubber or latex. The needle shield within the white or orange cap of the single-dose prefilled SureClick® autoinjectors and the gray needle cap of the single-dose prefilled syringes contain dry natural rubber.
-
Pregnant or plan to become pregnant. It is not known if AIMOVIG will harm your unborn baby.
- Breastfeeding or plan to breastfeed. It is not known if AIMOVIG passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while using AIMOVIG.
How should I take AIMOVIG? -
See the detailed “Instructions for Use” on complete information on how to take AIMOVIG.
- Take AIMOVIG exactly as your healthcare provider tells you to take it.
- Before you inject, always check the label of your single-dose prefilled autoinjector or single-dose prefilled syringe to make sure you have the correct medicine and the correct dose of AIMOVIG.
- AIMOVIG is injected under your skin (subcutaneously) 1 time each month.
- AIMOVIG comes in 2 different types of devices: a single-dose (1 time) prefilled autoinjector or a single-dose (1 time) prefilled syringe. Your healthcare provider will prescribe the type and dose that is best for you.
If you forget to take AIMOVIG or are not able to take the dose at the regular time, take your missed dose as soon as you remember. After that, you can continue to take AIMOVIG 1 time each month from the date of your last dose.
What are possible side effects of AIMOVIG?
AIMOVIG may cause serious side effects, including:
- Allergic reactions. Allergic reactions, including rash or swelling can happen after receiving AIMOVIG. This can happen within hours to days after using AIMOVIG. Call your healthcare provider or get emergency medical help right away if you have any of the following symptoms of an allergic reaction:
- swelling of the face, mouth, tongue or throat
- trouble breathing
- swelling of the face, mouth, tongue or throat
- Constipation with serious complications. Severe constipation can happen after receiving AIMOVIG. In some cases, people have been hospitalized or needed surgery. Contact your healthcare provider if you have severe constipation.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of AIMOVIG. Ask your pharmacist or healthcare provider for more information.
Call your healthcare provider for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088. You may also report side effects to Amgen at 1-800-77-AMGEN (1-800-772-6436).How should I store AIMOVIG?
- Store AIMOVIG in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep AIMOVIG in the original carton. This will protect the medicine from light.
- After removing AIMOVIG from the refrigerator, it can be stored at room temperature between
68°F to 77°F (20°C to 25°C) for up to 7 days.
- Throw away AIMOVIG that has been left at room temperature for more than 7 days.
-
Do not freeze.
- Do not shake.
General information about the safe and effective use of AIMOVIG.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use AIMOVIG for a condition for which it is not prescribed. Do not give AIMOVIG to other people, even if they have the same symptoms that you have. It can harm them. You can ask your pharmacist or healthcare provider for information about AIMOVIG that is written for healthcare professionals.What are the ingredients in AIMOVIG? -
Active Ingredient: erenumab-aooe
- Inactive Ingredients: acetate, polysorbate 80, and sucrose
AIMOVIG® (erenumab-aooe)
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, CA 91320-1799 U.S.A.
U.S. License No. 1080
Marketed by:
Amgen Inc. (Thousand Oaks, CA 91320), and
Novartis Pharmaceuticals Corporation (East Hanover, NJ 07936)
Patent: http://pat.amgen.com/aimovig/
© 2018-2019 Amgen Inc. All rights reserved.
For more information, go to www.aimovig.com or call 1-800-77-AMGEN (1-800-772-6436).This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 10/2019
[part number] v3
-
Allergic to rubber or latex. The needle shield within the white or orange cap of the single-dose prefilled SureClick® autoinjectors and the gray needle cap of the single-dose prefilled syringes contain dry natural rubber.
-
INSTRUCTIONS FOR USE
Instructions for Use
AIMOVIG® (AIM-oh-vig) (erenumab-aooe)
Injection, For Subcutaneous Use
Single-Dose Prefilled SureClick® Autoinjector
70 mg/mL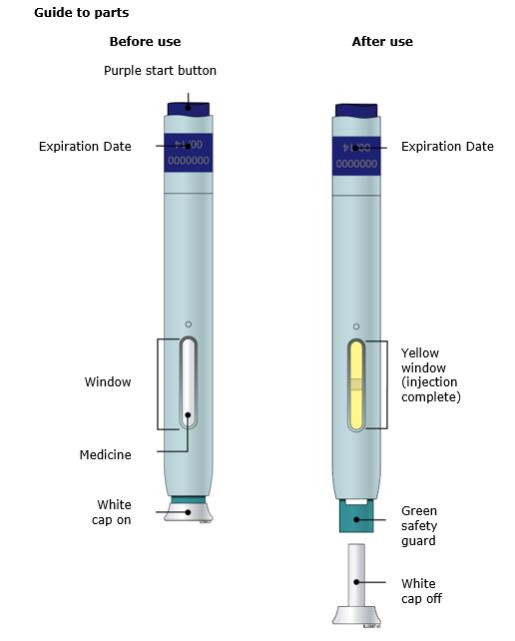
Important: Needle is inside the green safety guard.
Important
Before you use AIMOVIG SureClick autoinjector, read this important information:
Storing your AIMOVIG SureClick autoinjector
- Keep the autoinjector out of the reach of children.
- Keep the autoinjector in the original carton to protect from light.
- The autoinjector should be kept in the refrigerator at 36°F to 46°F (2°C to 8°C).
- After removing AIMOVIG from the refrigerator, it can be stored at room temperature between 68°F to 77°F (20°C to 25°C) for up to 7 days.
- Throw away AIMOVIG that has been left at room temperature for more than 7 days.
- Do not freeze.
Using your AIMOVIG SureClick autoinjector
- The needle shield within the white cap of the AIMOVIG autoinjector contains dry natural rubber, which is made from latex. Tell your healthcare provider if you are allergic to latex.
- It is important that you do not try to give the injection unless you or your caregiver has received training from your healthcare provider.
-
Do not use the autoinjector after the expiration date on the label.
-
Do not shake the autoinjector.
-
Do not remove the white cap from the autoinjector until you are ready to inject.
-
Do not freeze or use the autoinjector if it has been frozen.
- Do not use the autoinjector if it has been dropped on a hard surface. Part of the autoinjector may be broken even if you cannot see the break. Use a new autoinjector, and call 1-800-77-AMGEN (1-800-772-6436).
Step 1: Prepare
AIMOVIG comes as a single-dose (1 time) prefilled autoinjector. Your healthcare provider will prescribe the dose that is best for you.
Before you inject, always check the label of your single-dose prefilled autoinjector to make sure you have the correct medicine and the correct dose of AIMOVIG.
A Remove autoinjector from the carton.
Carefully lift the autoinjector straight up out of the carton.
Leave the autoinjector at room temperature for at least 30 minutes before injecting.
-
Do not put the autoinjector back in the refrigerator after it has reached room temperature.
-
Do not try to warm the autoinjector by using a heat source such as hot water or microwave.
-
Do not leave the autoinjector in direct sunlight.
-
Do not shake the autoinjector.
- Do not remove the white cap from the autoinjector yet.
B Inspect the autoinjector.
Make sure the medicine in the window is clear and colorless to slightly yellow.
-
Do not use the autoinjector if the medicine is cloudy or discolored or contains flakes or particles.
-
Do not use the autoinjector if any part appears cracked or broken.
-
Do not use the autoinjector if the autoinjector has been dropped.
-
Do not use the autoinjector if the white cap is missing or not securely attached.
- Do not use the autoinjector if the expiration date printed on the label has passed.
In all cases, use a new autoinjector, and call 1-800-77-AMGEN (1-800-772-6436).
C Gather all materials needed for your injection.
Wash your hands thoroughly with soap and water.
On a clean, well-lit work surface, place the:
- New autoinjector
- Alcohol wipes
- Cotton balls or gauze pads
- Adhesive bandages
- Sharps disposal container (See “Step 4: Finish”)
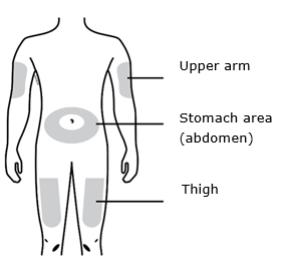
D Prepare and clean your injection site.
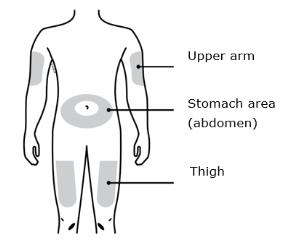
Only use these injection sites:
- Your thigh
- Stomach area (abdomen), except for a two inch area right around your navel
- Outer area of upper arm (only if someone else is giving you the injection)
Clean your injection site with an alcohol wipe. Let your skin dry.
-
Do not touch this area again before injecting.
-
Do not inject into areas where the skin is tender, bruised, red, or hard.
- Avoid injecting directly into raised, thick, red, or scaly skin patch or lesion, or areas with scars or stretch marks.
Step 2: Get ready
E Pull the white cap straight off, only when you are ready to inject. Do not leave the white cap off for more than five minutes. This can dry out the medicine.
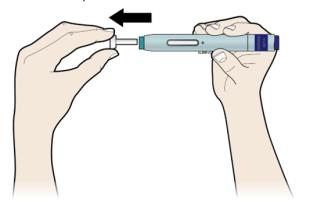
It is normal to see a drop of liquid at the end of the needle or green safety guard.
-
Do not twist or bend the white cap.
-
Do not put the white cap back onto the autoinjector.
-
Do not put fingers into the green safety guard.
- Do not remove the white cap from the autoinjector until you are ready to inject.
F Create a firm surface at the selected injection site (thigh, stomach, or outer areas of the upper arm), by using either the Stretch method or the Pinch method.
Stretch method
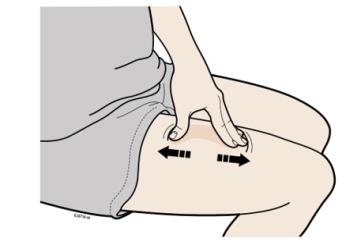
Stretch the skin firmly by moving your thumb and fingers in opposite directions, creating an area about two inches wide. 
Pinch method
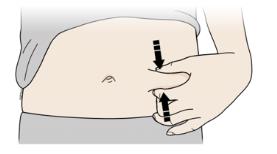
Pinch the skin firmly between your thumb and fingers, creating an area about two inches wide.
Important: It is important to keep skin stretched or pinched while injecting.
Step 3: Inject
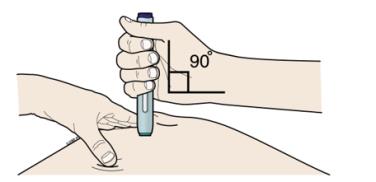
G Keep holding the stretched or pinched skin. With the white cap off, put the green safety guard on your skin at 90 degrees. The needle is inside the green safety guard. Do not touch the purple start button yet.
H Firmly push the autoinjector down onto skin until the autoinjector stops moving.
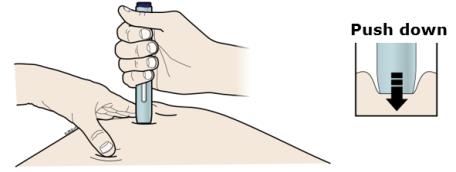
Important: You must push all the way down but do not touch the purple start button until you are ready to inject.
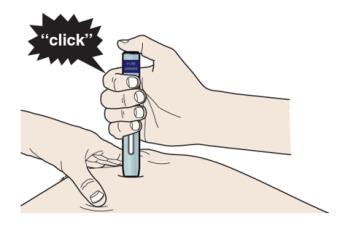
I When you are ready to inject, press the purple start button. You will hear a click.
J Keep pushing down on your skin. Then lift your thumb while still holding the autoinjector on your skin. Your injection could take about 15 seconds.
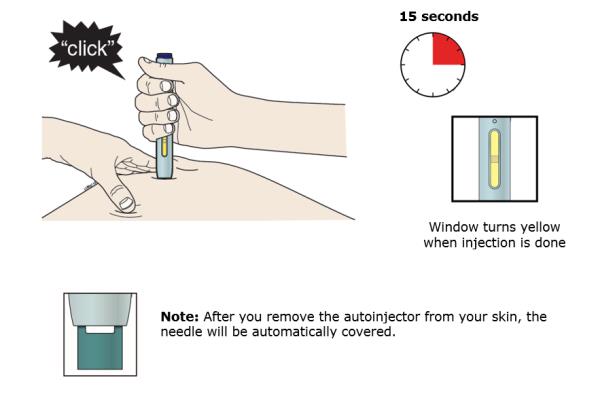
Important: When you remove the autoinjector, if the window has not turned yellow, or if it looks like the medicine is still injecting, this means you have not received a full dose. Call your healthcare provider immediately.
Step 4: Finish
K Discard the used autoinjector and the white cap.
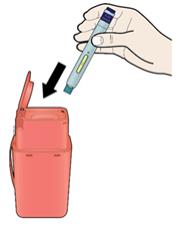
Put the used AIMOVIG autoinjector and white cap in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the SureClick autoinjector in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- Made of a heavy-duty plastic
- Can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- Upright and stable during use
- Leak-resistant
- Properly labeled to warn of hazardous waste inside the container
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal
-
Do not reuse the autoinjector.
- Do not recycle the autoinjector or sharps disposal container or throw them into household trash.
Important: Always keep the sharps disposal container out of the reach of children.
L Examine the injection site.
If there is blood, press a cotton ball or gauze pad on your injection site. Do not rub the injection site. Apply an adhesive bandage if needed.
Commonly asked questions
What will happen if I press the purple start button before I am ready to do the injection on my skin? Even when you press the purple start button, the injection will only happen when the green safety guard is also pushed into the autoinjector. Can I move the autoinjector around on my skin while I am choosing an injection site? It is okay to move the autoinjector around on the injection site as long as you do not press the purple start button. However, if you press the purple start button and the green safety guard is pushed into the autoinjector, the injection will begin. Can I release the purple start button after I start my injection? You can release the purple start button, but continue to hold the autoinjector firmly against your skin during the injection. Will the purple start button pop up after I release my thumb? The purple start button may not pop up after you release your thumb if you held your thumb down during the injection. This is okay. What do I do if I did not hear a click after pushing the device down on my skin for 15 seconds? If you did not hear a click, you can confirm a complete injection by checking that the window has turned yellow. Whom do I contact if I need help with the autoinjector or my injection? If you need more information or help, visit www.aimovig.com or call 1-800-77-AMGEN (1-800-772-6436). This Instructions for Use has been approved by the U.S. Food and Drug Administration.
AIMOVIG® (erenumab-aooe)
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, CA 91320-1799 USA
U.S. License No. 1080Marketed by:
Amgen Inc. (Thousand Oaks, CA 91320), and
Novartis Pharmaceuticals Corporation (East Hanover, NJ 07936)© 2018-2020 Amgen Inc. All rights reserved.
<partnumber> Revised: 02/2020 v3
Symbol table

This product contains dry natural rubber
Do not reuse

CAUTION,
consult accompanying documents

Lot number

- Keep the autoinjector out of the reach of children.
-
INSTRUCTIONS FOR USE
Instructions for Use
AIMOVIG® (AIM-oh-vig) (erenumab-aooe)
Injection, For Subcutaneous Use
Single-Dose Prefilled SureClick® Autoinjector
140 mg/mL
Important: Needle is inside the yellow safety guard.
Important
Before you use AIMOVIG SureClick autoinjector, read this important information:
Storing your AIMOVIG SureClick autoinjector
- Keep the autoinjector out of the reach of children.
- Keep the autoinjector in the original carton to protect from light.
- The autoinjector should be kept in the refrigerator at 36°F to 46°F (2°C to 8°C).
- After removing AIMOVIG from the refrigerator, it can be stored at room temperature between 68°F to 77°F (20°C to 25°C) for up to 7 days.
- Throw away AIMOVIG that has been left at room temperature for more than 7 days.
- Do not freeze.
Using your AIMOVIG SureClick autoinjector
- The needle shield within the orange cap of the AIMOVIG autoinjector contains dry natural rubber, which is made from latex. Tell your healthcare provider if you are allergic to latex.
- It is important that you do not try to give the injection unless you or your caregiver has received training from your healthcare provider.
-
Do not use the autoinjector after the expiration date on the label.
-
Do not shake the autoinjector.
-
Do not remove the orange cap from the autoinjector until you are ready to inject.
-
Do not freeze or use the autoinjector if it has been frozen.
- Do not use the autoinjector if it has been dropped on a hard surface. Part of the autoinjector may be broken even if you cannot see the break. Use a new autoinjector, and call 1-800-77-AMGEN (1-800-772-6436).
Step 1: Prepare
AIMOVIG comes as a single-dose (1 time) prefilled autoinjector. Your healthcare provider will prescribe the dose that is best for you.
Before you inject, always check the label of your single-dose prefilled autoinjector to make sure you have the correct medicine and the correct dose of AIMOVIG.
A Remove autoinjector from the carton.
Carefully lift the autoinjector straight up out of the carton.
Leave the autoinjector at room temperature for at least 30 minutes before injecting.
-
Do not put the autoinjector back in the refrigerator after it has reached room temperature.
-
Do not try to warm the autoinjector by using a heat source such as hot water or microwave.
-
Do not leave the autoinjector in direct sunlight.
-
Do not shake the autoinjector.
- Do not remove the orange cap from the autoinjector yet.
B Inspect the autoinjector.
Make sure the medicine in the window is clear and colorless to slightly yellow.
-
Do not use the autoinjector if the medicine is cloudy or discolored or contains flakes or particles.
-
Do not use the autoinjector if any part appears cracked or broken.
-
Do not use the autoinjector if the autoinjector has been dropped.
-
Do not use the autoinjector if the orange cap is missing or not securely attached.
- Do not use the autoinjector if the expiration date printed on the label has passed.
In all cases, use a new autoinjector, and call 1-800-77-AMGEN (1-800-772-6436).
C Gather all materials needed for your injection.
Wash your hands thoroughly with soap and water.
On a clean, well-lit work surface, place the:
- New autoinjector
- Alcohol wipes
- Cotton balls or gauze pads
- Adhesive bandages
- Sharps disposal container (See “Step 4: Finish”)
D Prepare and clean your injection site.
Only use these injection sites:
- Your thigh
- Stomach area (abdomen), except for a two inch area right around your navel
- Outer area of upper arm (only if someone else is giving you the injection)
Clean your injection site with an alcohol wipe. Let your skin dry.
-
Do not touch this area again before injecting.
-
Do not inject into areas where the skin is tender, bruised, red, or hard.
- Avoid injecting directly into raised, thick, red, or scaly skin patch or lesion, or areas with scars or stretch marks.
Step 2: Get ready
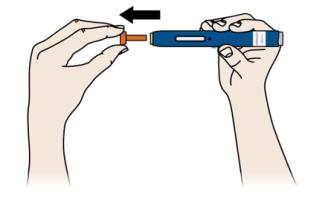
E Pull the orange cap straight off, only when you are ready to inject. Do not leave the orange cap off for more than five minutes. This can dry out the medicine.

It is normal to see a drop of liquid at the end of the needle or yellow safety guard.
-
Do not twist or bend the orange cap.
-
Do not put the orange cap back onto the autoinjector.
-
Do not put fingers into the yellow safety guard.
- Do not remove the orange cap from the autoinjector until you are ready to inject.
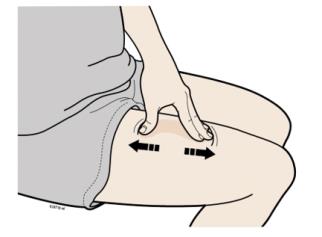
F Create a firm surface at the selected injection site (thigh, stomach, or outer areas of the upper arm), by using either the Stretch method or the Pinch method.
Stretch method

Stretch the skin firmly by moving your thumb and fingers in opposite directions, creating an area about two inches wide.
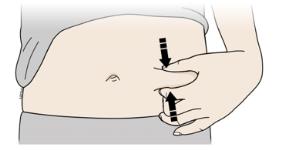
Pinch method

Pinch the skin firmly between your thumb and fingers, creating an area about two inches wide.
Important: it is important to keep skin stretched or pinched while injecting.
Step 3: Inject
G Keep holding the stretched or pinched skin. With the orange cap off, put the yellow safety guard on your skin at 90 degrees. The needle is inside the yellow safety guard. Do not touch the gray start button yet.
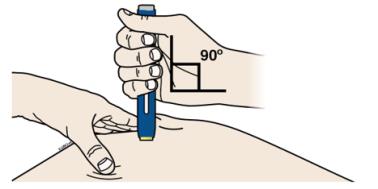
H Firmly push the autoinjector down onto skin until the autoinjector stops moving.
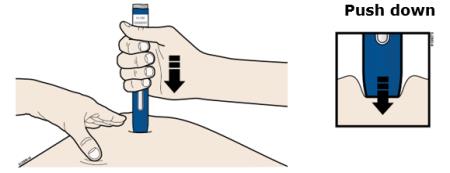
Important: You must push all the way down but do not touch the gray start button until you are ready to inject.
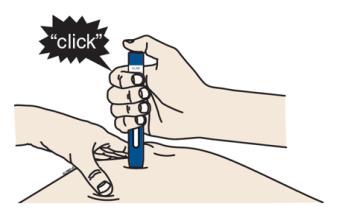
I When you are ready to inject, press the gray start button. You will hear a click.
J Keep pushing down on your skin. Then lift your thumb while still holding the autoinjector on your skin. Your injection could take about 15 seconds.
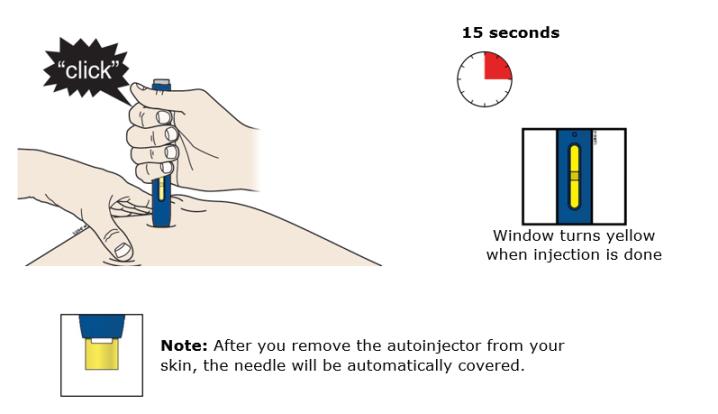
Important: When you remove the autoinjector, if the window has not turned yellow, or if it looks like the medicine is still injecting, this means you have not received a full dose. Call your healthcare provider immediately.
Step 4: Finish
K Discard the used autoinjector and the orange cap.
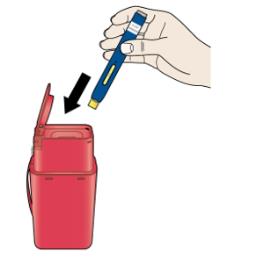
Put the used AIMOVIG autoinjector and orange cap in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the SureClick autoinjector in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- Made of a heavy-duty plastic
- Can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- Upright and stable during use
- Leak-resistant
- Properly labeled to warn of hazardous waste inside the container
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal
-
Do not reuse the autoinjector.
- Do not recycle the autoinjector or sharps disposal container or throw them into household trash.
Important: Always keep the sharps disposal container out of the reach of children.
L Examine the injection site.
If there is blood, press a cotton ball or gauze pad on your injection site. Do not rub the injection site. Apply an adhesive bandage if needed.
Commonly asked questions
What will happen if I press the gray start button before I am ready to do the injection on my skin? Even when you press the gray start button, the injection will only happen when the yellow safety guard is also pushed into the autoinjector. Can I move the autoinjector around on my skin while I am choosing an injection site? It is okay to move the autoinjector around on the injection site as long as you do not press the gray start button. However, if you press the gray start button and the yellow safety guard is pushed into the autoinjector, the injection will begin. Can I release the gray start button after I start my injection? You can release the gray start button, but continue to hold the autoinjector firmly against your skin during the injection. Will the gray start button pop up after I release my thumb? The gray start button may not pop up after you release your thumb if you held your thumb down during the injection. This is okay. What do I do if I did not hear a click after pushing the device down on my skin for 15 seconds? If you did not hear a click, you can confirm a complete injection by checking that the window has turned yellow. Whom do I contact if I need help with the autoinjector or my injection? If you need more information or help, visit www.aimovig.com or call 1-800-77-AMGEN (1-800-772-6436). This Instructions for Use has been approved by the U.S. Food and Drug Administration.
AIMOVIG® (erenumab-aooe)
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, CA 91320-1799 USA
U.S. License No. 1080Marketed by:
Amgen Inc. (Thousand Oaks, CA 91320), and
Novartis Pharmaceuticals Corporation (East Hanover, NJ 07936)© 2019-2020 Amgen Inc. All rights reserved.
<partnumber> Revised: 02/2020 v2
Symbol table

This product contains dry natural rubber
Do not reuse

CAUTION,
consult accompanying documents

Lot number
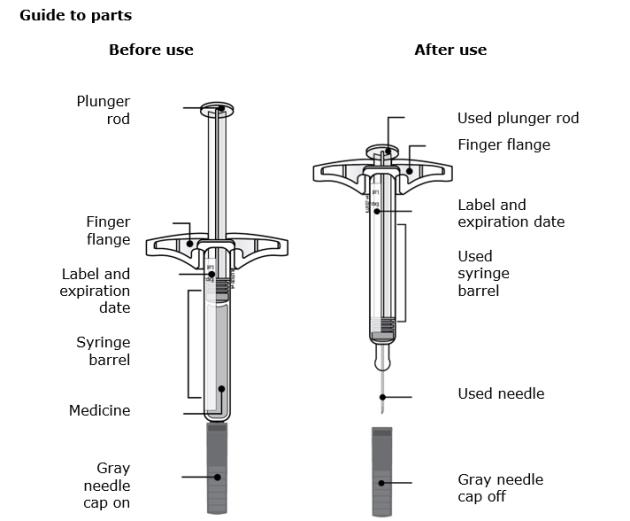
Instructions for Use
AIMOVIG® (AIM-oh-vig) (erenumab-aooe)
Injection, For Subcutaneous Use
Single-Dose Prefilled Syringe
70 mg/mL and 140 mg/mL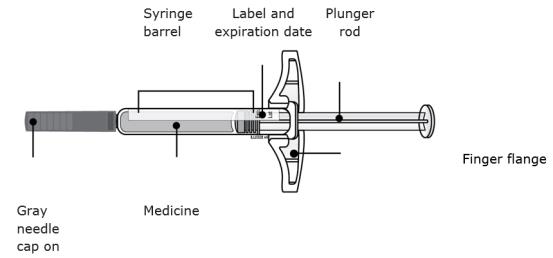
Important: Needle is inside the gray needle cap.
Important
Before you use AIMOVIG prefilled syringe, read this important information:
Storing your AIMOVIG prefilled syringe
- Keep the syringe out of the reach of children.
- Keep the syringe in the original carton to protect from light.
- The syringe should be kept in the refrigerator at 36°F to 46°F (2°C to 8°C).
- After removing AIMOVIG from the refrigerator, it can be stored at room temperature between 68°F to 77°F (20°C to 25°C) for up to 7 days.
- Throw away AIMOVIG that has been left at room temperature for more than 7 days.
- Do not freeze.
Using your AIMOVIG prefilled syringe
- The gray needle cap of the prefilled syringe contains dry natural rubber, which is made from latex. Tell your healthcare provider if you are allergic to latex.
- It is important that you do not try to give the injection unless you or your caregiver has received training from your healthcare provider.
-
Do not use a syringe after the expiration date on the label.
-
Do not shake the syringe.
-
Do not remove the gray needle cap from the syringe until you are ready to inject.
-
Do not use the syringe if it has been frozen.
- Do not use a syringe if it has been dropped on a hard surface. Part of the syringe may be broken even if you cannot see the break. Use a new syringe, and call 1-800-77-AMGEN (1-800-772-6436).
Step 1: Prepare
AIMOVIG comes as a single-dose (1 time) prefilled syringe. Your healthcare provider will prescribe the dose that is best for you.
Before you inject, always check the label of your single-dose prefilled syringe to make sure you have the correct medicine and the correct dose of AIMOVIG.
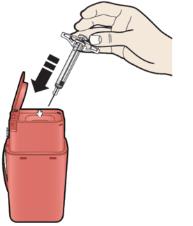
A Remove AIMOVIG prefilled syringe from the carton.
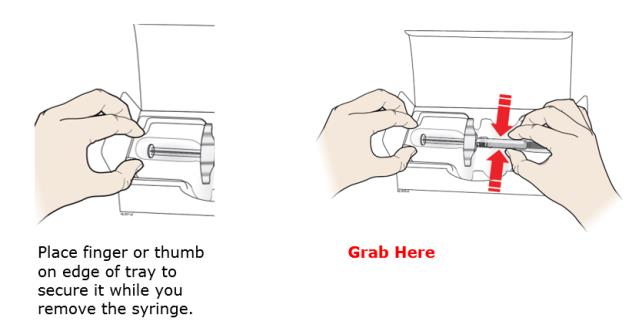
Grab the syringe barrel to remove the syringe from the tray.
For safety reasons:
-
Do not grab the plunger rod.
-
Do not grab the gray needle cap.
-
Do not remove the gray needle cap until you are ready to inject.
- Do not remove the finger flange. This is part of the syringe.
Leave the syringe at room temperature for at least 30 minutes before injecting.
-
Do not put the syringe back in the refrigerator after it has reached room temperature.
-
Do not try to warm the syringe by using a heat source such as hot water or microwave.
-
Do not leave the syringe in direct sunlight.
- Do not shake the syringe.
Important: Always hold the prefilled syringe by the syringe barrel.
B Inspect the AIMOVIG prefilled syringe.
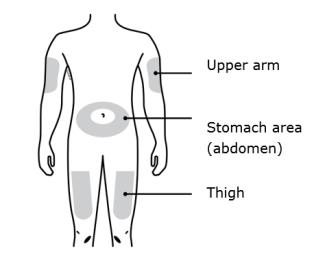
Always hold the syringe by the syringe barrel.
Make sure the medicine in the syringe is clear and colorless to slightly yellow.
-
Do not use the syringe if the medicine is cloudy or discolored or contains flakes or particles.
-
Do not use the syringe if any part appears cracked or broken.
-
Do not use the syringe if the syringe has been dropped.
-
Do not use the syringe if the gray needle cap is missing or not securely attached.
- Do not use the syringe if the expiration date printed on the label has passed.
In all cases, use a new syringe, and call 1-800-77-AMGEN (1-800-772-6436).
C Gather all materials needed for your injection.
Wash your hands thoroughly with soap and water.
On a clean, well-lit work surface, place the:
- New syringe
- Alcohol wipes
- Cotton balls or gauze pads
- Adhesive bandages
- Sharps disposal container
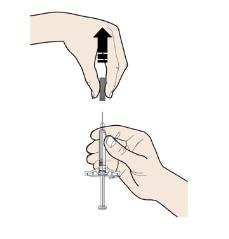
D Prepare and clean your injection site.
Only use these injection sites:
- Your thigh
- Stomach area (abdomen), except for a two inch area right around your navel
- Outer area of upper arm (only if someone else is giving you the injection)
Clean your injection site with an alcohol wipe. Let your skin dry.
-
Do not touch this area again before injecting.
- Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid injecting directly into raised, thick, red, or scaly skin patch or lesion, or areas with scars or stretch marks.
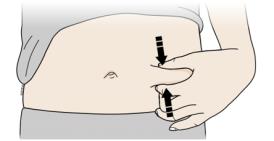
Step 2: Get ready
E Pull gray needle cap straight out and away from your body, only when you are ready to inject. Do not leave the gray needle cap off for more than five minutes. This can dry out the medicine.
It is normal to see a drop of liquid at the end of the needle.
-
Do not twist or bend the gray needle cap.
-
Do not put the gray needle cap back onto the syringe.
- Do not remove the gray needle cap from the syringe until you are ready to inject.
Important: Throw the gray needle cap into the sharps disposal container.
F Pinch your injection site to create a firm surface.
Pinch skin firmly between your thumb and fingers, creating an area about two inches wide.
Important: Keep skin pinched while injecting.
Step 3: Inject
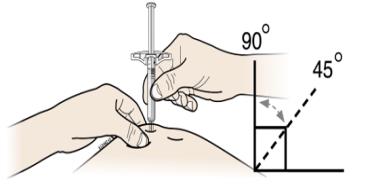
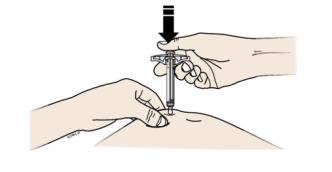
G Hold the pinch. With the gray needle cap off, insert the syringe into your skin at 45 to 90 degrees.
Do not place your finger on the plunger rod while inserting the needle.
H Place your finger on the plunger rod. Using slow and constant pressure, push the plunger rod all the way down until the prefilled syringe stops moving.
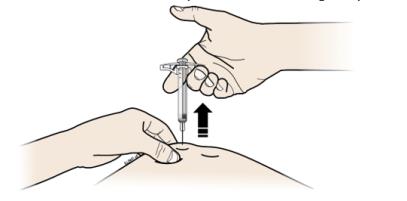
I When done, release your thumb, and gently lift the syringe off of your skin.

Important: When you remove the syringe, if it looks like the medicine is still in the syringe barrel, this means you have not received a full dose. Call your healthcare provider immediately.
Step 4: Finish
J Discard the used syringe and the gray needle cap.

Put the used AIMOVIG syringe and gray needle cap in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the syringe in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- Made of a heavy-duty plastic
- Can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- Upright and stable during use
- Leak-resistant
- Properly labeled to warn of hazardous waste inside the container
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
-
Do not reuse the syringe.
- Do not recycle the syringe or sharps disposal container or throw them into household trash.
Important: Always keep the sharps disposal container out of the reach of children.
K Examine the injection site.
If there is blood, press a cotton ball or gauze pad on your injection site. Do not rub the injection site. Apply an adhesive bandage if needed.
For more information, go to www.aimovig.com or call 1-800-77-AMGEN (1-800-772-6436).
AIMOVIG® (erenumab-aooe)
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, CA 91320-1799 USA
U.S. License No. 1080Marketed by:
Amgen Inc. (Thousand Oaks, CA 91320), and
Novartis Pharmaceuticals Corporation (East Hanover, NJ 07936)© 2018-2020 Amgen Inc. All rights reserved.
Symbol table

This product contains dry natural rubber
Do not reuse
CAUTION,
consult accompanying documents
Lot number
This Instructions for Use has been approved by the U.S. Food and Drug Administration.Approved: 02/2020
[partnumber] v3
- Keep the autoinjector out of the reach of children.
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
1 x 70 mg/mL Prefilled Autoinjector
NDC: 55513-841-01
Rx Only
AMGEN®
NOVARTIS
aimovig®
(erenumab-aooe)
Injection
70 mg/mL
70 mg/mL
Prefilled Autoinjector
For Subcutaneous Use Only
Store at 2°C to 8°C (36°F to 46°F) in original carton
to protect from light. Do Not Freeze. Do Not Shake.
Discard Any Unused Portion.
Keep out of the sight and reach of children.
For more information, go to Aimovig.com or
call 1-800-77-AMGEN (1-800-772-6436).
CAUTION, Consult
Accompanying Documents
Do Not Reuse
This Product Contains
Dry Natural Rubber.
No U.S. standard of potency
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
1 x 70 mg/mL Prefilled Syringe
NDC: 55513-840-01
Rx Only
AMGEN®
NOVARTIS
aimovig®
(erenumab-aooe)
Injection
70 mg/mL
70 mg/mL
Single-dose Prefilled Syringe
For Subcutaneous Use Only
Store at 2°C to 8°C (36°F to 46°F) in original carton to protect
from light. Do Not Freeze. Do Not Shake. Discard Any Unused Portion.
Keep out of the sight and reach of children.
For more information, go to Aimovig.com or
call 1-800-77-AMGEN (1-800-772-6436).
CAUTION, Consult
Accompanying Documents
Do Not Reuse
This Product Contains
Dry Natural Rubber.

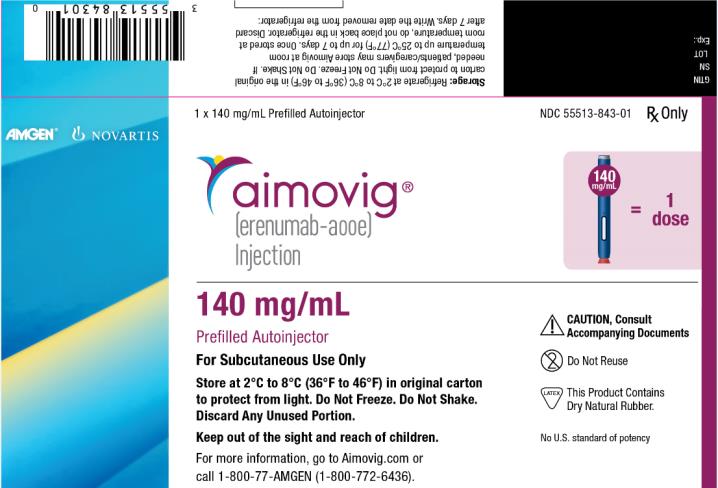
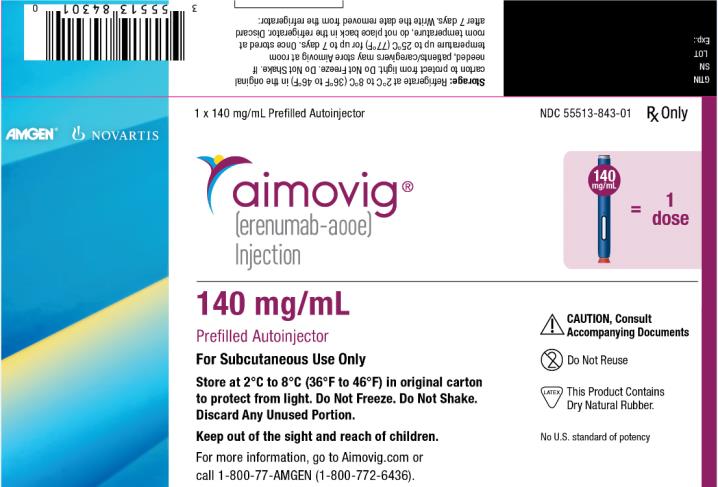
PRINCIPAL DISPLAY PANEL
1 x 140 mg/mL Prefilled Autoinjector
NDC: 55513-843-01
Rx Only
AMGEN®
NOVARTIS
aimovig®
(erenumab-aooe)
Injection
140 mg/mL = 1 dose
140 mg/mL
Prefilled Autoinjector
For Subcutaneous Use Only
Store at 2°C to 8°C (36°F to 46°F) in original carton
to protect from light. Do Not Freeze. Do Not Shake.
Discard Any Unused Portion.
Keep out of the sight and reach of children.
For more information, go to Aimovig.com or
call 1-800-77-AMGEN (1-800-772-6436).
CAUTION, Consult
Accompanying Documents
Do Not Reuse
This Product Contains
Dry Natural Rubber.
No U.S. standard of potency
PRINCIPAL DISPLAY PANEL
1 x 140 mg/mL Prefilled Syringe
NDC: 55513-842-01
Rx Only
AMGEN®
NOVARTIS
aimovig®
(erenumab-aooe)
Injection
140 mg/mL = 1 dose
140 mg/mL
Single-dose Prefilled Syringe
For Subcutaneous Use Only
Store at 2°C to 8°C (36°F to 46°F) in original carton to protect
from light. Do Not Freeze. Do Not Shake. Discard Any Unused Portion.
Keep out of the sight and reach of children.
For more information, go to Aimovig.com or
call 1-800-77-AMGEN (1-800-772-6436).
CAUTION, Consult
Accompanying Documents
Do Not Reuse
This Product Contains
Dry Natural Rubber.

-
INGREDIENTS AND APPEARANCE
AIMOVIG
erenumab-aooe injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55513-840 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ERENUMAB (UNII: I5I8VB78VT) (ERENUMAB - UNII:I5I8VB78VT) ERENUMAB 70 mg in 1 mL Inactive Ingredients Ingredient Name Strength ACETATE ION (UNII: 569DQM74SC) 1.5 mg in 1 mL SUCROSE (UNII: C151H8M554) 73 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.10 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55513-840-01 1 in 1 CARTON 05/22/2018 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC: 55513-840-02 2 in 1 CARTON 05/22/2018 01/31/2021 2 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761077 05/22/2018 AIMOVIG
erenumab-aooe injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55513-841 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ERENUMAB (UNII: I5I8VB78VT) (ERENUMAB - UNII:I5I8VB78VT) ERENUMAB 70 mg in 1 mL Inactive Ingredients Ingredient Name Strength ACETATE ION (UNII: 569DQM74SC) 1.5 mg in 1 mL SUCROSE (UNII: C151H8M554) 73 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.10 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55513-841-01 1 in 1 CARTON 05/22/2018 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC: 55513-841-02 2 in 1 CARTON 05/22/2018 01/31/2021 2 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761077 05/22/2018 AIMOVIG
erenumab-aooe injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55513-843 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ERENUMAB-AOOE (UNII: I5I8VB78VT) (ERENUMAB-AOOE - UNII:I5I8VB78VT) ERENUMAB-AOOE 140 mg in 1 mL Inactive Ingredients Ingredient Name Strength ACETATE ION (UNII: 569DQM74SC) 2 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.10 mg in 1 mL SUCROSE (UNII: C151H8M554) 65 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55513-843-01 1 in 1 CARTON 03/15/2019 1 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761077 03/15/2019 AIMOVIG
erenumab-aooe injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55513-842 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ERENUMAB-AOOE (UNII: I5I8VB78VT) (ERENUMAB-AOOE - UNII:I5I8VB78VT) ERENUMAB-AOOE 140 mg in 1 mL Inactive Ingredients Ingredient Name Strength ACETATE ION (UNII: 569DQM74SC) 2 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.10 mg in 1 mL SUCROSE (UNII: C151H8M554) 65 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55513-842-01 1 in 1 CARTON 03/15/2019 1 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761077 03/15/2019 Labeler - Amgen Inc (039976196) Establishment Name Address ID/FEI Business Operations Amgen Manufacturing Ltd 785800020 ANALYSIS(55513-840, 55513-841, 55513-843, 55513-842) , LABEL(55513-840, 55513-841, 55513-843, 55513-842) , MANUFACTURE(55513-840, 55513-841, 55513-843, 55513-842) , PACK(55513-840, 55513-841, 55513-843, 55513-842) Establishment Name Address ID/FEI Business Operations Amgen Technology (ireland) Unlimited Company 896293920 ANALYSIS(55513-840, 55513-841, 55513-843, 55513-842) Establishment Name Address ID/FEI Business Operations Amgen, Inc 039976196 ANALYSIS(55513-840, 55513-841, 55513-843, 55513-842) Establishment Name Address ID/FEI Business Operations BioReliance Corporation 147227730 ANALYSIS(55513-840, 55513-841, 55513-843, 55513-842)
Trademark Results [AIMOVIG]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AIMOVIG 87325932 5536605 Live/Registered |
Amgen Inc. 2017-02-06 |
 AIMOVIG 87079789 5530809 Live/Registered |
Amgen Inc. 2016-06-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.