CHAPSTICK LIPSHIELD 365- avobenzone, dimethicone, homosalate, octinoxate, octisalate, oxybenzone stick
CHAPSTICK LIPSHIELD 365 by
Drug Labeling and Warnings
CHAPSTICK LIPSHIELD 365 by is a Otc medication manufactured, distributed, or labeled by Pfizer Consumer Healthcare, Fareva Richmond, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
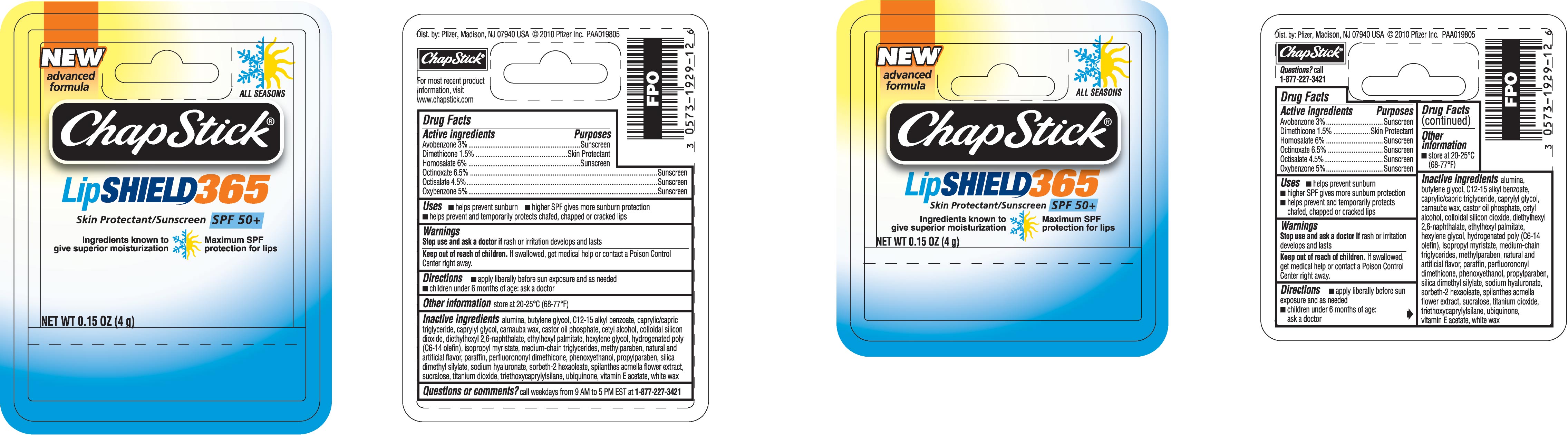
- DRUG FACTS
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
- DIRECTIONS
- OTHER INFORMATION
-
INACTIVE INGREDIENTS
alumina, butylene glycol, C12-15 alkyl benzoate, caprylic/capric triglyceride, caprylyl glycol, carnauba wax, castor oil phosphate, cetyl alcohol, colloidal silicon dioxide, diethylhexyl 2,6-naphthalate, ethylhexyl palmitate, hexylene glycol, hydrogenated poly (C6-14 olefin), isopropyl myristate, medium-chain triglycerides, methylparaben, natural and artificial flavor, paraffin, perfluorononyl dimethicone, phenoxyethanol, propylparaben, silica dimethyl silyate, sodium hyaluronate, sorbeth-2 hexaoleate, spilanthes acmella flower extract, sucralose, titanium dioxide, triethoxycaprylysilane, ubiquinone, vitamin E acetate, white wax
- QUESTIONS OR COMMENTS?
-
PRODUCT PACKAGING
The product packaging shown below represents a sample of that currently in use. Additional packaging may also be available.
Skin Protectant/Sunscreen SPF 50+
Ingredients known to give superior moisturization
Maximum SPF protection for lips
Dist. by: Pfizer, Madison, NJ 07940 USA © 2010 Pfizer Inc.
For most recent product information, visit www.chapstick.com

-
INGREDIENTS AND APPEARANCE
CHAPSTICK LIPSHIELD 365
avobenzone, dimethicone, homosalate, octinoxate, octisalate, oxybenzone stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0573-1929 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 g DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 15 mg in 1 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 60 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 65 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIETHYLHEXYL 2,6-NAPHTHALATE (UNII: I0DQJ7YGXM) ETHYLHEXYL PALMITATE (UNII: 2865993309) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) METHYLPARABEN (UNII: A2I8C7HI9T) PARAFFIN (UNII: I9O0E3H2ZE) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLPARABEN (UNII: Z8IX2SC1OH) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ACMELLA OLERACEA FLOWER (UNII: 2794N5KM0K) SUCRALOSE (UNII: 96K6UQ3ZD4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WHITE WAX (UNII: 7G1J5DA97F) Product Characteristics Color WHITE (off white) Score Shape BULLET (Cylindrical) Size 42mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0573-1929-12 1 in 1 BLISTER PACK 1 4 g in 1 CYLINDER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 07/01/2011 Labeler - Pfizer Consumer Healthcare (828831730) Establishment Name Address ID/FEI Business Operations Fareva Richmond, Inc. 969523245 ANALYSIS, LABEL, MANUFACTURE, PACK, RELABEL, REPACK
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.