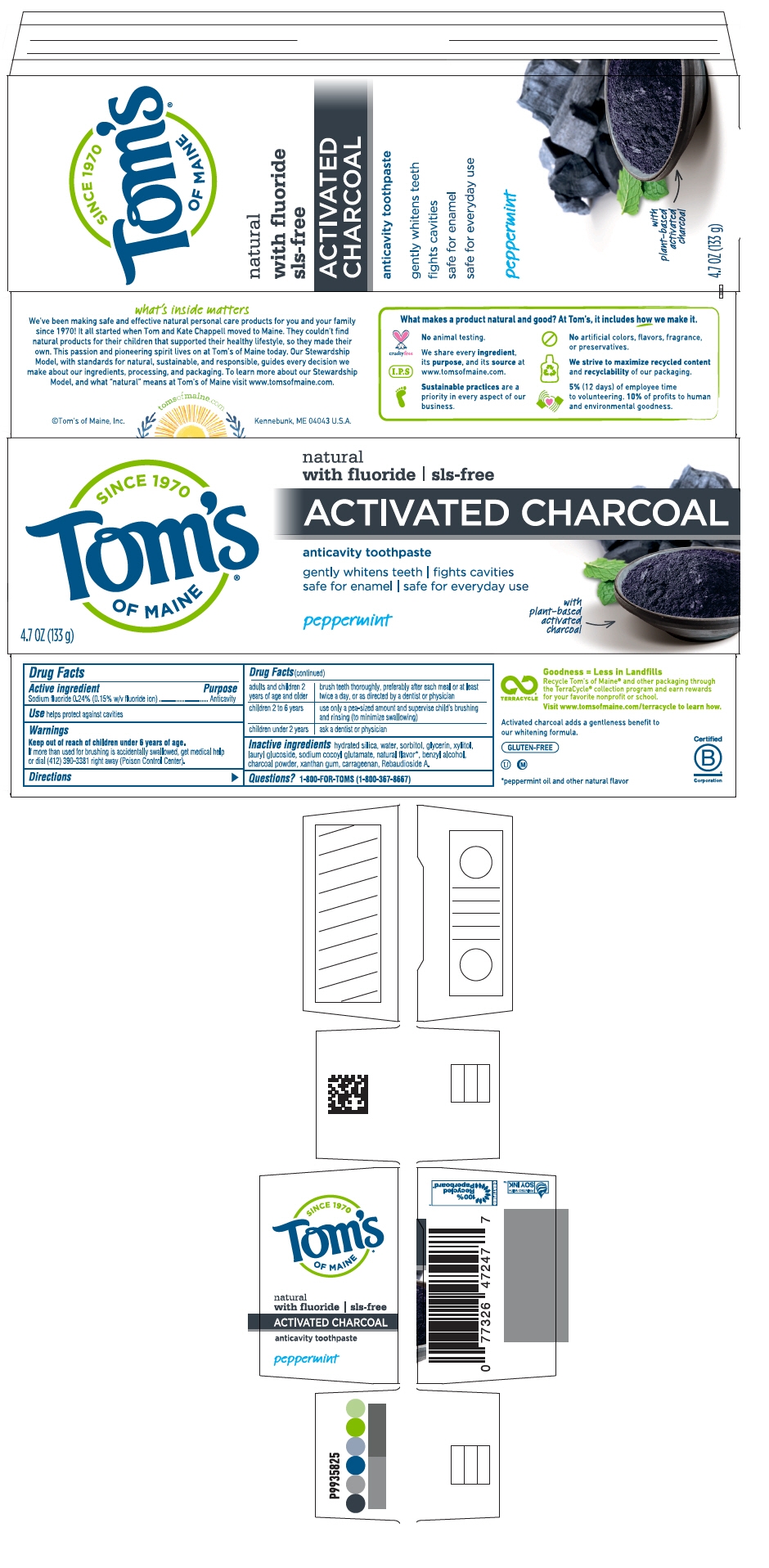
PEPPERMINT CHARCOAL ANTICAVITY PCFT6 GENTLY WHITENS TEETH / FIGHTS CAVITIES / SAFE FOR ENAMEL / SAFE FOR EVERYDAY USE- sodium fluoride paste, dentifrice
Peppermint Charcoal Anticavity PCFT6 by
Drug Labeling and Warnings
Peppermint Charcoal Anticavity PCFT6 by is a Otc medication manufactured, distributed, or labeled by Tom's of Maine, Inc., Accupac, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
adults and children 2 years of age and older brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician children 2 to 6 years use only a pea-sized amount and supervise child's brushing and rinsing (to minimize swallowing) children under 2 years ask a dentist or physician - Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 133 g Tube Carton
-
INGREDIENTS AND APPEARANCE
PEPPERMINT CHARCOAL ANTICAVITY PCFT6 GENTLY WHITENS TEETH / FIGHTS CAVITIES / SAFE FOR ENAMEL / SAFE FOR EVERYDAY USE
sodium fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 51009-900 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Fluoride (UNII: 8ZYQ1474W7) (fluoride ion - UNII:Q80VPU408O) fluoride ion 0.0024 g in 1 g Inactive Ingredients Ingredient Name Strength Hydrated Silica (UNII: Y6O7T4G8P9) Water (UNII: 059QF0KO0R) Sorbitol (UNII: 506T60A25R) Glycerin (UNII: PDC6A3C0OX) Xylitol (UNII: VCQ006KQ1E) Lauryl Glucoside (UNII: 76LN7P7UCU) Sodium Cocoyl Glutamate (UNII: BMT4RCZ3HG) Peppermint Oil (UNII: AV092KU4JH) Benzyl Alcohol (UNII: LKG8494WBH) Activated Charcoal (UNII: 2P3VWU3H10) Xanthan Gum (UNII: TTV12P4NEE) Carrageenan (UNII: 5C69YCD2YJ) Rebaudioside A (UNII: B3FUD0528F) Product Characteristics Color BLACK Score Shape Size Flavor PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51009-900-47 1 in 1 CARTON 12/18/2018 1 133 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 51009-900-01 1 in 1 CARTON 11/15/2019 2 0.75 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC: 51009-900-10 0.75 g in 1 TUBE; Type 0: Not a Combination Product 01/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part355 12/18/2018 Labeler - Tom's of Maine, Inc. (052764354) Establishment Name Address ID/FEI Business Operations Tom's of Maine, Inc. 829517189 MANUFACTURE(51009-900)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.