Terravitals BakNek by Terravitals LLC
Terravitals BakNek by
Drug Labeling and Warnings
Terravitals BakNek by is a Homeopathic medication manufactured, distributed, or labeled by Terravitals LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TERRAVITALS BAKNEK- antimonium crudum, antimonium tartaricum, arnica montana, cuprum metallicum, lycopodium clavatum pellet
Terravitals LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
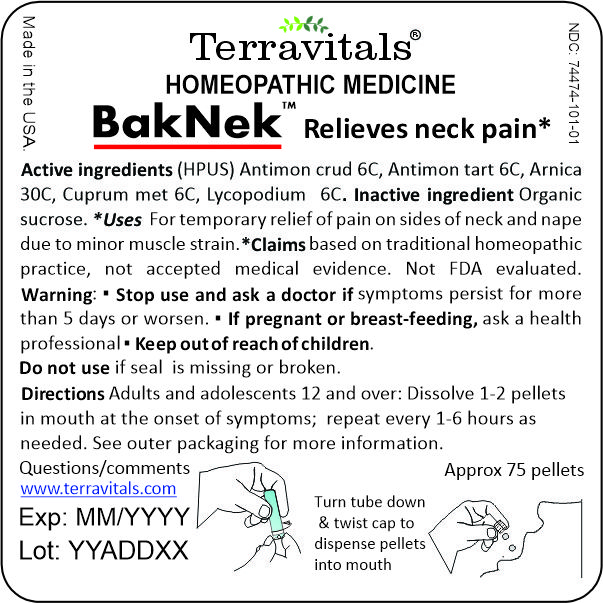
Terravitals BakNek Active Ingredients
Drug Facts
Active ingredients
Antimonium crudum 6C HPUS
Antimonium tartaricum 6C HPUS
Arnica montana 30C HPUS
Cuprum metallicum 6C HPUS
Lycopodium clavatum 6C HPUS
'HPUS' indicates that this ingredient is officially included in the Homeopathic Pharmacopoeia of the United States.
Small inner packaging label:
Terravitals BakNek Purpose
Active ingredients** ...................................................................... Purpose
Antimonium crudum 6C HPUS...............neck/back pain, neck/nape cramp
Antimonium tartaricum 6C HPUS .............. stiffness aggravated by motion
Arnica montana 30C HPUS................weakness and painful glands of neck
Cuprum metallicum 6C HPUS.. swelling neck glands,muscles back to neck
Lycopodium clavatum 6C HPUS.............painful stiffness of left side of neck
'HPUS' indicates that this ingredient is officially included in the Homeopathic Pharmacopoeia of the United States
**'C' is homeopathic dilution.
Uses* For temporary relief of pain on sides of neck and nape due to minor muscle strain.
*Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
Terravitals BakNek Warnings
Warnings Stop use and ask a doctor If symptoms persist more than 5 days or worsen. If pregnant or breastfeeding, ask a health professional before use. Keep out of reach of children.
Directions: Adults and adolescents 12 and over: Dissolve 1-2 pellets in mouth at the onset of symptoms, repeat every 1- 6 hours as needed
Terravitals BakNek Other
Other information: Store in a dry place between 15-30°C (59-86°F) and out of direct sunlight.
Terravitals BakNek Inactive Ingredient
Inactive ingredient Organic Sucrose certified by DE-OKO-070. (Organic logo)
Terravitals BakNek main panel
TERRAVITALS BakNek
Homeopathic Medicine
Relieves neck pain*
NDC: 74474-101-01
Approx 75 Oral soluble pellets
Non-drowsy
Not habit-forming
No drug interactions
No known side effects
Made in the USA by Terravitals LLC, Gaithersburg MD 20879
www.terravitals.com
Exp: MM/YYYY
Lot: YYADXX
Outer packaging label:

| TERRAVITALS BAKNEK
antimonium crudum, antimonium tartaricum, arnica montana, cuprum metallicum, lycopodium clavatum pellet |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Terravitals LLC (117322434) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Terravitals LLC | 117322434 | manufacture(74474-101) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.