UNISHIELD EXTRA STRENGTH NON-ASPIRIN- acetaminophen tablet UNISHIELD EXTRA STRENGTH NON-ASPIRIN- acetaminophen tablet, film coated UNISHIELD EXTRA STRENGTH NON ASPIRIN- acetaminophen tablet
Unishield Extra Strength Non-Aspirin by
Drug Labeling and Warnings
Unishield Extra Strength Non-Aspirin by is a Otc medication manufactured, distributed, or labeled by Unishield, Unifirst First Aid Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
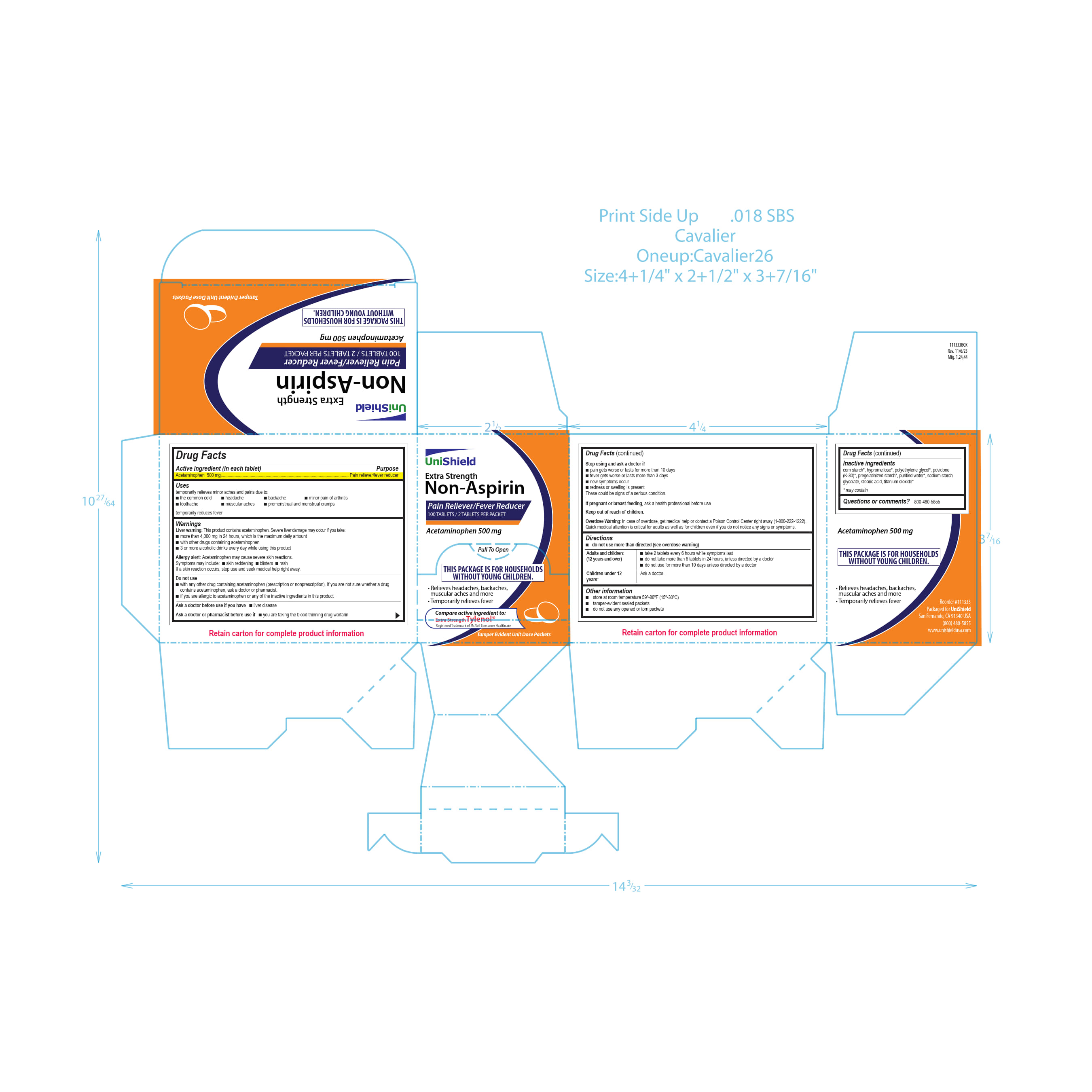
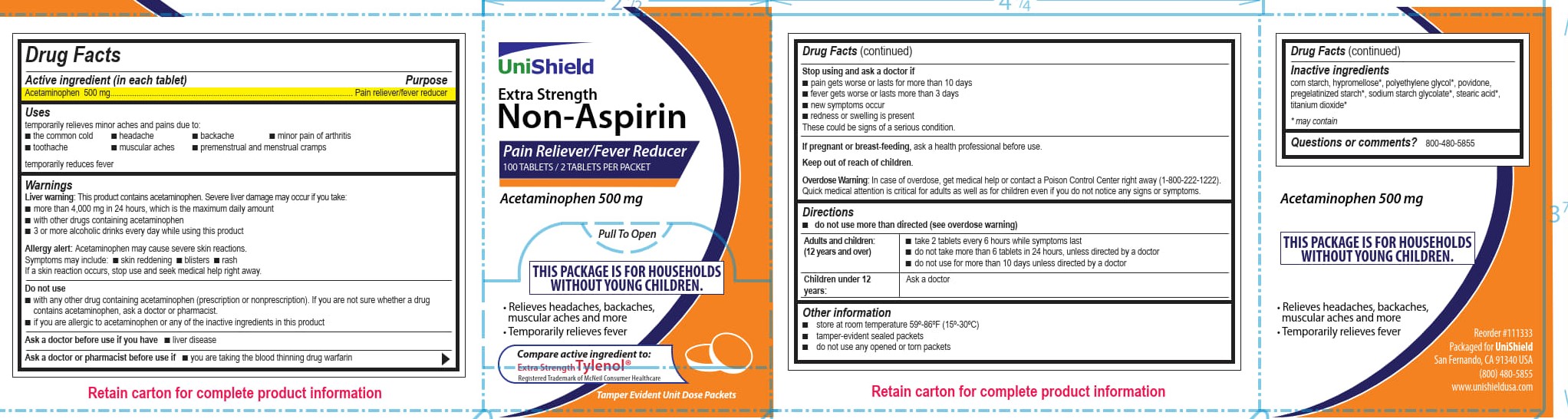
WARNINGS
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
■ more than 4,000 mg in 24 hours, which is the maximum daily amount
■ with other drugs containing acetaminophen
■ 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
■ skin reddening
■ blisters
■ rash
If a skin reaction occurs, stop use and seek medical help right away.
- DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
■ do not use more than directed (see overdose warning)
Adults and children 12 years and over
■ take 2 tablets every 6 hours while symptoms last.
■ do not take more than 6 tablets in 24 hours, unless directed by a doctor■ do not use for more than 10 days unless directed by a doctor
Children under 12 years Ask a doctor
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
-
Unishield Extra Strength Non-Aspirin
Unishield
Extra Strength
Non-Aspirin
Pain Reliever/Fever Reducer
100 Tablets/2Tablets Per Packet
Acetaminophen 500 mg
Pull To Open
This Package is for Households Without Young Children.
Relieves headaches, backaches, muscular aches and more
Temporarily relieves fever
Compare active ingredients to:
Extra Strength Tylenol®
Registered Trademark of McNeil Consumer Healthcare
Tamper Evident Unit Dose Packets
-
Unishield Extra Strength Non-Aspirin Label
Unishield
Extra Strength
Non-Aspirin
Pain Reliever/Fever Reducer
100 Tablets/2Tablets Per Packet
Acetaminophen 500 mg
Pull To Open
This Package is for Households Without Young Children.
Relieves headaches, backaches, muscular aches and more
Temporarily relieves fever
Compare active ingredients to:
Extra Strength Tylenol®
Registered Trademark of McNeil Consumer Healthcare
Tamper Evident Unit Dose Packets

-
INGREDIENTS AND APPEARANCE
UNISHIELD EXTRA STRENGTH NON-ASPIRIN
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49314-1773 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) Product Characteristics Color white Score 2 pieces Shape ROUND Size 12mm Flavor Imprint Code 44;148 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49314-1773-3 50 in 1 BOX 06/01/2023 1 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 06/01/2023 UNISHIELD EXTRA STRENGTH NON-ASPIRIN
acetaminophen tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49314-1753 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) POVIDONE (UNII: FZ989GH94E) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) HYPROMELLOSES (UNII: 3NXW29V3WO) MALTODEXTRIN (UNII: 7CVR7L4A2D) STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color white Score no score Shape ROUND Size 12mm Flavor Imprint Code FR;33 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49314-1753-3 50 in 1 BOX 03/01/2021 11/01/2023 1 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 03/01/2021 11/01/2023 UNISHIELD EXTRA STRENGTH NON-ASPIRIN
acetaminophen tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49314-1763 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) HYPROMELLOSES (UNII: 3NXW29V3WO) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color white Score no score Shape ROUND Size 12mm Flavor Imprint Code AZ;235 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49314-1763-3 50 in 1 BOX 03/01/2021 1 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 03/01/2021 UNISHIELD EXTRA STRENGTH NON ASPIRIN
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49314-1783 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POVIDONE K30 (UNII: U725QWY32X) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color white Score no score Shape ROUND Size 12mm Flavor Imprint Code G552 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49314-1783-3 50 in 1 BOX 10/01/2025 1 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 10/01/2025 Labeler - Unishield (790677053) Registrant - Unifirst First Aid Corporation (832947092) Establishment Name Address ID/FEI Business Operations LNK International, Inc, 832867837 manufacture(49314-1773)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.