ALLERGY- acetaminophen, chlorpheniramine maleate, phenylephrine hcl tablet
Allergy by
Drug Labeling and Warnings
Allergy by is a Otc medication manufactured, distributed, or labeled by L. Perrigo Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
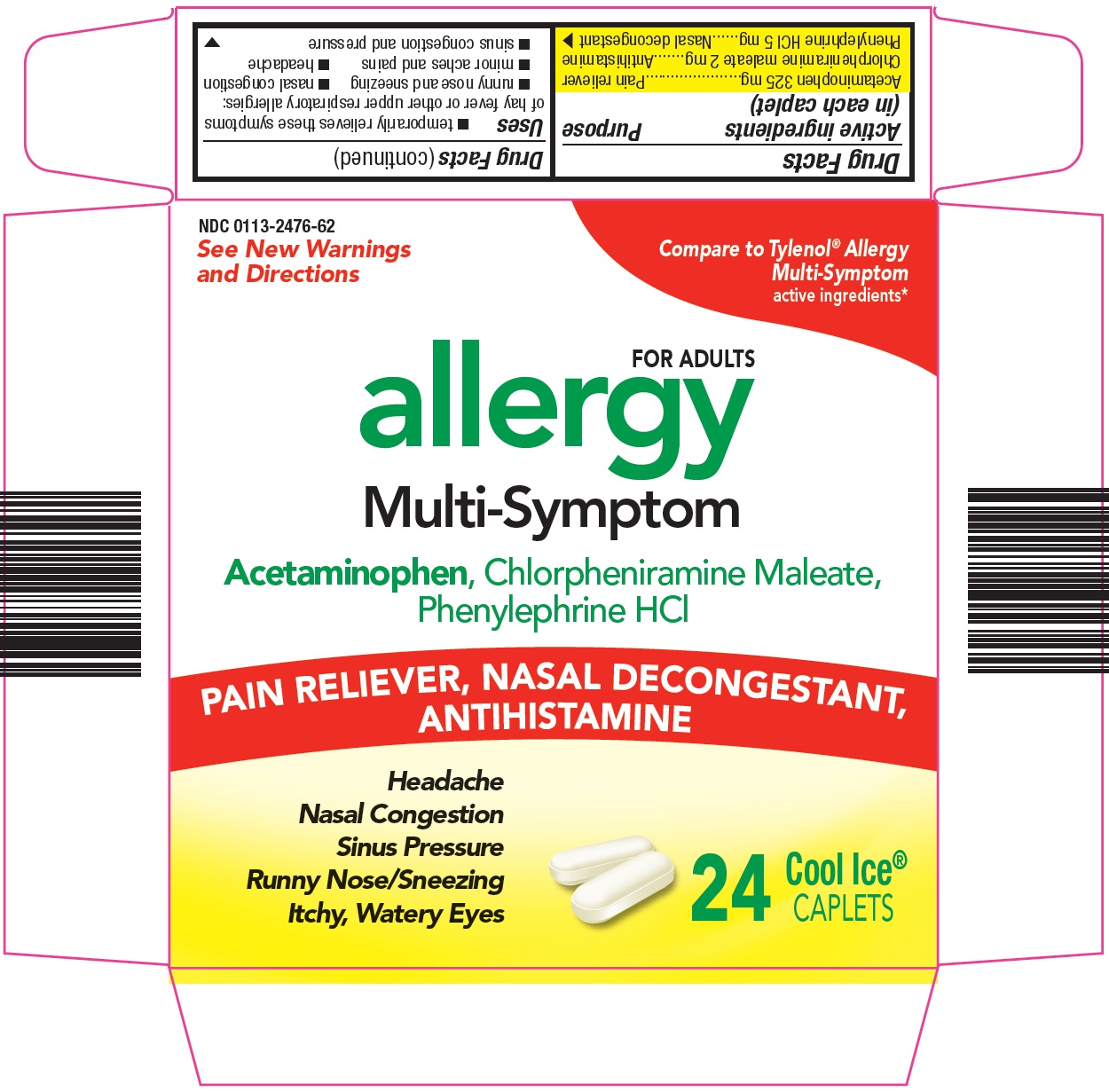
- Active ingredients (in each caplet)
- Purpose
-
Uses
- temporarily relieves these symptoms of hay fever or other upper respiratory allergies:
- runny nose and sneezing
- nasal congestion
- minor aches and pains
- headache
- sinus congestion and pressure
- temporarily relieves these additional symptoms of hay fever:
- itching of the nose or throat
- itchy, watery eyes
- helps clear nasal passages
- helps decongest sinus openings and passages
-
Warnings
Liver warning: This product contains acetaminophen. The maximum daily dose of this product is 10 caplets (3,250 mg acetaminophen) in 24 hours. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
- to make a child sleepy
Ask a doctor before use if you have
- liver disease
- heart disease
- diabetes
- high blood pressure
- thyroid disease
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- do not exceed recommended dosage
- excitability may occur, especially in children
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic drinks
- be careful when driving a motor vehicle or operating machinery
- drowsiness may occur
- Directions
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
See New Warnings and Directions
Compare to Tylenol® Allergy Multi-Symptom active ingredients
FOR ADULTS
allergy
Multi-Symptom
Acetaminophen, Chlorpheniramine Maleate, Phenylephrine HCl
PAIN RELIEVER, NASAL DECONGESTANT, ANTIHISTAMINE
Headache
Nasal Congestion
Sinus Pressure
Runny Nose/Sneezing
Itchy, Watery Eyes
24 Cool Ice® CAPLETS
-
INGREDIENTS AND APPEARANCE
ALLERGY
acetaminophen, chlorpheniramine maleate, phenylephrine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0113-2476 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 2 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color WHITE (off white) Score no score Shape CAPSULE Size 19mm Flavor VANILLA (Vanilla-menthol) Imprint Code L476 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0113-2476-62 12 in 1 CARTON 03/20/2014 1 2 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 03/20/2014 Labeler - L. Perrigo Company (006013346)
Trademark Results [Allergy]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY 97069656 not registered Live/Pending |
Rodriguez, Kent J 2021-10-12 |
 ALLERGY 90819383 not registered Live/Pending |
Rodriguez, Kent J 2021-07-09 |
 ALLERGY 87534443 5406627 Live/Registered |
RSM Medical Inc. 2017-07-19 |
 ALLERGY 87518600 5400551 Live/Registered |
RSM Medical Inc. 2017-07-06 |
 ALLERGY 74392251 not registered Dead/Abandoned |
Danta, Inc. 1993-05-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.