Ciclopirox by Sun Pharmaceutical Industries, Inc. / Sun Pharma Canada Inc. CICLOPIROX suspension
Ciclopirox by
Drug Labeling and Warnings
Ciclopirox by is a Prescription medication manufactured, distributed, or labeled by Sun Pharmaceutical Industries, Inc., Sun Pharma Canada Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Ciclopirox Topical Suspension USP, 0.77% is for topical use.
Each gram of Ciclopirox Topical Suspension USP contains 7.70 mg of ciclopirox (as ciclopirox olamine) in a water miscible suspension base consisting of cetyl alcohol, cocamide DEA, lactic acid, mineral oil, myristyl alcohol, octyldodecanol, polysorbate 60, purified water, sorbitan monostearate, stearyl alcohol, and benzyl alcohol (1%) as preservative.
Ciclopirox Topical Suspension USP contains a synthetic, broad-spectrum, antifungal agent ciclopirox (as ciclopirox olamine). The chemical name is 6-cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone, 2-aminoethanol salt.
The CAS Registry Number is 41621-49-2.
The chemical structure is:
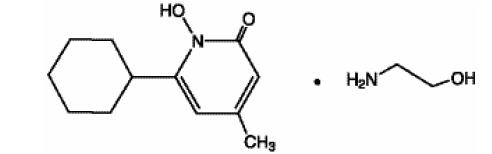
Ciclopirox Topical Suspension USP has a pH of 7.
-
CLINICAL PHARMACOLOGY
Ciclopirox is a broad-spectrum, antifungal agent that inhibits the growth of pathogenic dermatophytes, yeasts, and Malassezia furfur. Ciclopirox exhibits fungicidal activity in vitro against isolates of Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, Microsporum canis, and Candida albicans.
Pharmacokinetic studies in men with radiolabeled ciclopirox solution in polyethylene glycol 400 showed an average of 1.3% absorption of the dose when it was applied topically to 750 cm2 on the back followed by occlusion for 6 hours. The biological half-life was 1.7 hours and excretion occurred via the kidney. Two days after application only 0.01% of the dose applied could be found in the urine. Fecal excretion was negligible. Autoradiographic studies with human cadaver skin showed that ciclopirox penetrates into the hair and through the epidermis and hair follicles into the sebaceous glands and dermis, while a portion of the drug remains in the stratum corneum.
In vitro penetration studies in frozen or fresh excised human cadaver and pig skin indicated that the penetration of Ciclopirox Topical Suspension USP is equivalent to that of Ciclopirox Cream USP. Therapeutic equivalence of cream and suspension formulations also was indicated by studies of experimentally induced guinea pig and human trichophytosis.
-
INDICATIONS AND USAGE
Ciclopirox Topical Suspension USP is indicated for the topical treatment of the following dermal infections: tinea pedis, tinea cruris, and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum canis; cutaneous candidiasis (moniliasis) due to Candida albicans; and tinea (pityriasis) versicolor due to Malassezia furfur.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
If a reaction suggesting sensitivity or chemical irritation should occur with the use of Ciclopirox Topical Suspension USP, treatment should be discontinued and appropriate therapy instituted.
Information for Patients
The patient should be told to:
- Use the medication for the full treatment time even though signs/symptoms may have improved and notify the physician if there is no improvement after four weeks.
- Inform the physician if the area of application shows signs of increased irritation (redness, itching, burning, blistering, swelling, oozing) indicative of possible sensitization.
- Avoid the use of occlusive wrappings or dressings.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A carcinogenicity study in female mice dosed cutaneously twice per week for 50 weeks followed by a 6-month drug-free observation period prior to necropsy revealed no evidence of tumors at the application site. The following in vitro and in vivo genotoxicity tests have been conducted with ciclopirox olamine: studies to evaluate gene mutation in the Ames Salmonella/Mammalian Microsome Assay (negative) and Yeast Saccharomyces Cerevisiae Assay (negative) and studies to evaluate chromosome aberrations in vivo in the Mouse Dominant Lethal Assay and in the Mouse Micronucleus Assay at 500 mg/kg (negative). The following battery of in vitro genotoxicity tests were conducted with ciclopirox: a chromosome aberration assay in V79 Chinese Hamster Cells, with and without metabolic activation (positive); a gene mutation assay in the HGPRT – test with V79 Chinese Hamster Cells (negative); and a primary DNA damage assay (i.e., unscheduled DNA Synthesis Assay in A549 Human Cells (negative)). An in vitro Cell Transformation Assay in BALB/C3T3 Cells was negative for cell transformation. In an in vivo Chinese Hamster Bone Marrow Cytogenetic Assay, ciclopirox was negative for chromosome aberrations at 5000 mg/kg.
Pregnancy Category B
Reproduction studies have been performed in the mouse, rat, rabbit, and monkey, via various routes of administration, at doses 10 times or more the topical human dose and have revealed no significant evidence of impaired fertility or harm to the fetus due to ciclopirox. There are, however, no adequate or well-controlled studies in pregnant women. Because animal studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
-
ADVERSE REACTIONS
In the controlled clinical trial with 89 patients using ciclopirox topical suspension and 89 patients using the vehicle, the incidence of adverse reactions were low. Those considered possibly related to treatment or occurring in more than one patient were pruritus, which occurred in two patients using ciclopirox topical suspension and one patient using the suspension vehicle, and burning, which occurred in one patient using ciclopirox topical suspension.
-
DOSAGE AND ADMINISTRATION
Gently massage Ciclopirox Topical Suspension USP, 0.77% into the affected and surrounding skin areas twice daily, in the morning and evening. Clinical improvement with relief of pruritus and other symptoms usually occurs within the first week of treatment. If a patient shows no clinical improvement after four weeks of treatment with Ciclopirox Topical Suspension USP, the diagnosis should be redetermined. Patients with tinea versicolor usually exhibit clinical and mycological clearing after two weeks of treatment.
-
HOW SUPPLIED
Ciclopirox Topical Suspension USP, 0.77% is supplied in 30 mL (NDC: 51672-1323-3) and 60 mL (NDC: 51672-1323-4) bottles.
Bottle space provided to allow for vigorous shaking before each use.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 60 mL Bottle Carton
60 mL
NDC: 51672-1323-4
Ciclopirox
Topical Suspension
USP, 0.77% (w/w)
FOR DERMATOLOGIC
USE ONLY.NOT FOR
OPHTHALMIC USE.Bottle space provided
to allow for vigorous
shaking before each use.Keep this and all
medications out of the
reach of children.Rx only
TARO

-
INGREDIENTS AND APPEARANCE
CICLOPIROX
ciclopirox suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51672-1323 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Ciclopirox Olamine (UNII: 50MD4SB4AP) (Ciclopirox - UNII:19W019ZDRJ) Ciclopirox 7.7 mg in 1 mL Inactive Ingredients Ingredient Name Strength benzyl alcohol (UNII: LKG8494WBH) cetyl alcohol (UNII: 936JST6JCN) coco diethanolamide (UNII: 92005F972D) lactic acid, unspecified form (UNII: 33X04XA5AT) mineral oil (UNII: T5L8T28FGP) myristyl alcohol (UNII: V42034O9PU) octyldodecanol (UNII: 461N1O614Y) polysorbate 60 (UNII: CAL22UVI4M) water (UNII: 059QF0KO0R) sorbitan monostearate (UNII: NVZ4I0H58X) stearyl alcohol (UNII: 2KR89I4H1Y) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51672-1323-3 1 in 1 CARTON 08/10/2005 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 51672-1323-4 1 in 1 CARTON 08/10/2005 2 60 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077092 08/10/2005 Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370) Establishment Name Address ID/FEI Business Operations Taro Pharmaceuticals Inc. 206263295 MANUFACTURE(51672-1323)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.