MUCUS- guaifenesin tablet, extended release
Mucus by
Drug Labeling and Warnings
Mucus by is a Otc medication manufactured, distributed, or labeled by MCKESSON (HEALTH MART), RECKITT BENCKISER HEALTHCARE INTERNATIONAL LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each extended-release tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
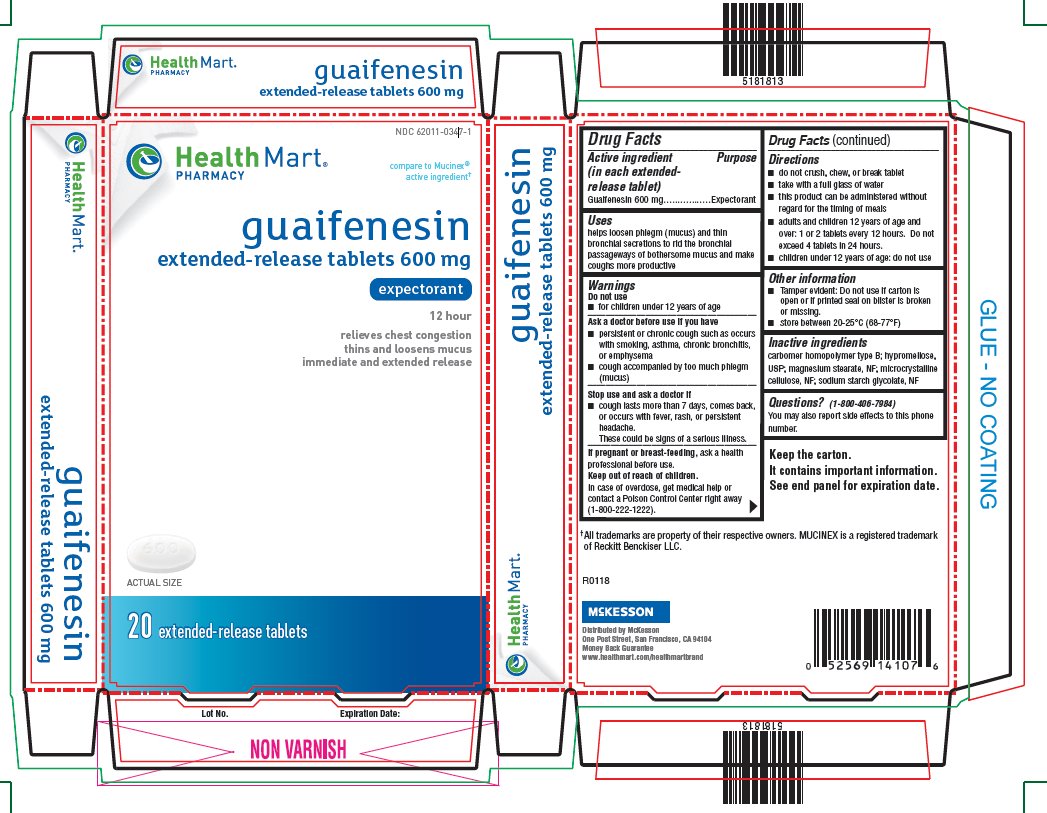
PRINCIPAL DISPLAY PANEL - 600 mg Tablet Blister Pack Carton
NDC: 62011-0347-1
Health Mart®
PHARMACYcompare to Mucinex®
active ingredient†guaifenesin
extended-release tablets 600 mgexpectorant
12 hour
relieves chest congestion
thins and loosens mucus
immediate and extended release20 extended-release tablets
-
INGREDIENTS AND APPEARANCE
MUCUS
guaifenesin tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62011-0347 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Guaifenesin (UNII: 495W7451VQ) (Guaifenesin - UNII:495W7451VQ) Guaifenesin 600 mg Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL OR ALLYL SUCROSE CROSSLINKED) (UNII: K6MOM3T5YL) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color WHITE Score no score Shape OVAL Size 16mm Flavor Imprint Code Mucinex;600 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62011-0347-1 1 in 1 CARTON 02/08/2018 1 20 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021282 02/08/2018 Labeler - MCKESSON (HEALTH MART) (177667227) Establishment Name Address ID/FEI Business Operations RECKITT BENCKISER HEALTHCARE INTERNATIONAL LTD 230780363 MANUFACTURE(62011-0347)
Trademark Results [Mucus]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MUCUS 97340231 not registered Live/Pending |
RB Health (US) LLC 2022-03-31 |
 MUCUS 87778407 not registered Live/Pending |
Reckitt Benckiser LLC 2018-01-31 |
 MUCUS 87778395 not registered Live/Pending |
Reckitt Benckiser LLC 2018-01-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.