Workvie Work Pain Relief Therapy Cream by Marketites LLC Workvie 4oz Cream
Workvie Work Pain Relief Therapy Cream by
Drug Labeling and Warnings
Workvie Work Pain Relief Therapy Cream by is a Otc medication manufactured, distributed, or labeled by Marketites LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
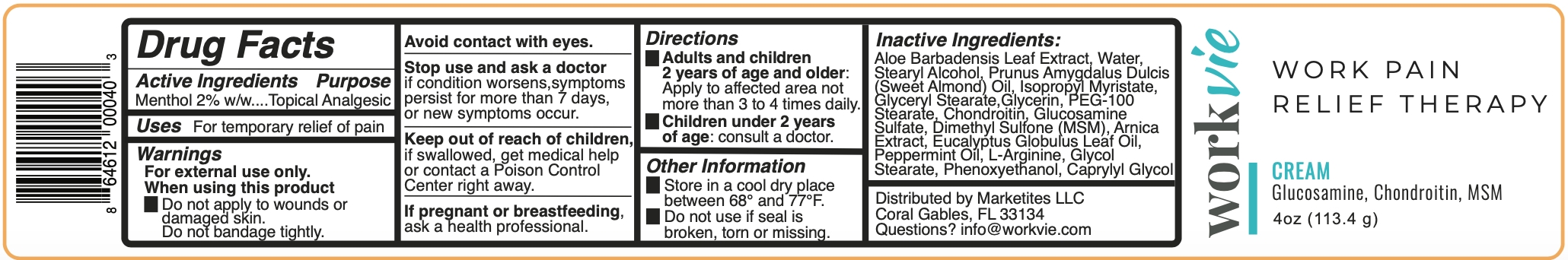
WORKVIE WORK PAIN RELIEF THERAPY CREAM- menthol cream
Marketites LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Workvie 4oz Cream
Warnings
Warnings
For external use only. Avoid contact with eyes.
Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily. Children under 2 years of age: consult a doctor.
Other Information
Store in a cool dry place between 68º and 77ºF.
Do not use if seal is broken, torn or missing.
Inactive Ingredients
Aloe Barbadensis Leaf Extract, Water, Stearyl Alcohol, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Isopropyl Myristate, Glyceryl Stearate, Glycerin, PEG-100 Stearate, Chondroitin, Glucosamine Sulfate, Dimethyl Sulfone (MSM), Arnica Extract, Eucalyptus Globulus Leaf Oil, Peppermint Oil, L-Arginine, Glycol Stearate, Phenoxyethanol, Caprylyl Glycol
| WORKVIE WORK PAIN RELIEF THERAPY CREAM
menthol cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Marketites LLC (036140212) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.