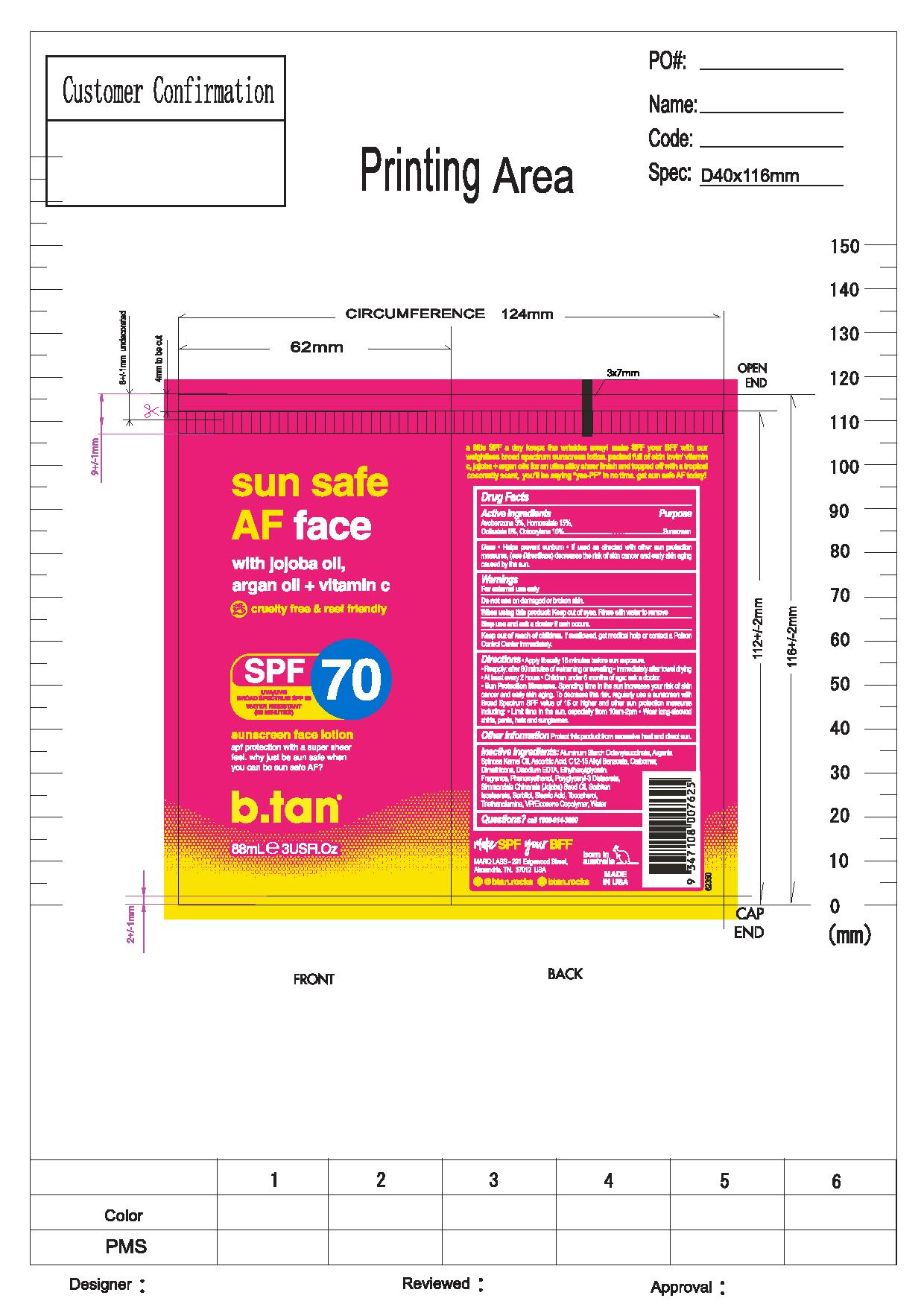
b.tan Sun Safe AF Face by Marque of Brands Americas, LLC b.tan Sun Safe AF
b.tan Sun Safe AF Face by
Drug Labeling and Warnings
b.tan Sun Safe AF Face by is a Otc medication manufactured, distributed, or labeled by Marque of Brands Americas, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
B.TAN SUN SAFE AF FACE SPF 70- avobenzone, homosalate, octisalate, octocrylene lotion
Marque of Brands Americas, LLC
----------
b.tan Sun Safe AF
- Helps prevent sunburn
- If used as directed with other sun protection measures, ( see Directions) decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- Apple liberally 15 minutes before sun exposure.
- Reapply: after 80 minutes of swimming or sweating
- Immediately after towel drying
- At least every 2 hours
- Children under 6 months of age: ask a doctor.
- Sun Protection Measures.
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10am-2pm
- Wear long-sleeved shirts, pants, hats and sunglasses.
Aluminum Starch Octenylsuccinate, Argania Spinosa Kernal Oil, Ascorbic Acid, C12-15 Alkyl Benzoate Carbomer, Dimethicone, Disodium EDTA, Ehtylhexylglycerin, Fragrance, Phenoxyethanol, Polyglyceryl-3 Distearate, Simmondsia Chinesis (Jojoba) Seed Oil, Sorbitan Isostearate, Sorbitol, Stearic Acid, Tocopherol, Triethanolamine, VP/ Eicosene Copolymer, Water.
| B.TAN SUN SAFE AF FACE
SPF 70
avobenzone, homosalate, octisalate, octocrylene lotion |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Marque of Brands Americas, LLC (118733944) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Marque of Brands Americas, LLC | 118733944 | manufacture(73978-004) | |
Revised: 8/2025
Document Id: 3d4739c4-ff73-cf2b-e063-6294a90aea2a
Set id: ba8874bf-fc0d-95e0-e053-2a95a90ae361
Version: 3
Effective Time: 20250826