Assured by Anhui NHU Pharmaceutical Co., Ltd. Drug Facts
Assured by
Drug Labeling and Warnings
Assured by is a Otc medication manufactured, distributed, or labeled by Anhui NHU Pharmaceutical Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
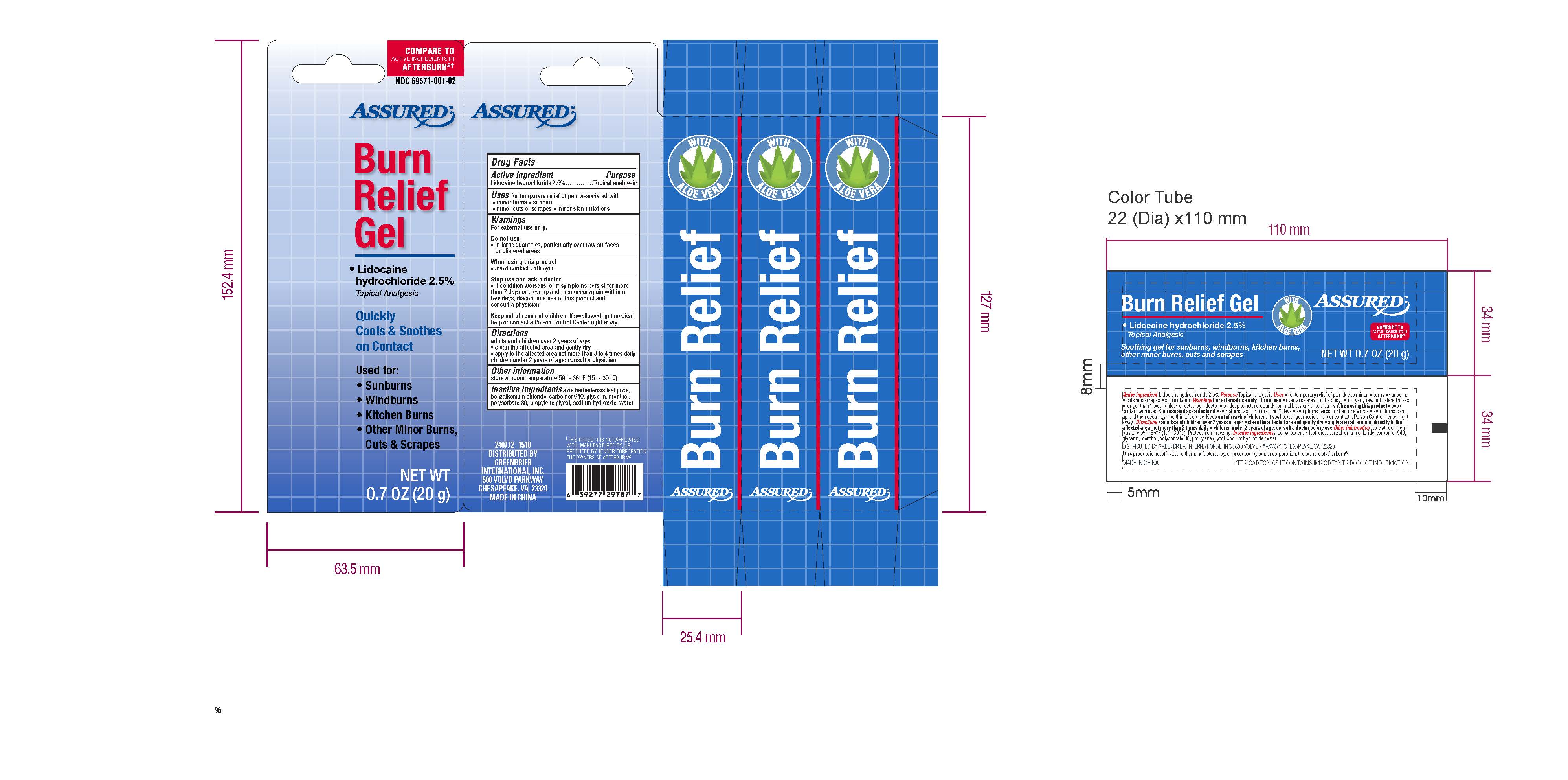
ASSURED- lidocaine hydrochloride gel
Anhui NHU Pharmaceutical Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Inactive Ingredients aloe barbadensis leaf juice,
benzalkonium chloride, carbomer 940, glycerin, menthol,
polysorbate 80, propylene glycol, sodium hydroxide, water
Uses for temporary relief of pain associated with
-minor burns -sunburn
-minor cuts or scrapes - minor skin irritations
Warnings
For external use only
Do not use
-in large quantities, particularly over raw surfaces
or blistered areas
When using this product
-avoid contact with eyes
Stop Use and ask a doctor
-if condition worsens, or if symptoms persist for more than
7 days or clear up and then occur again within a few
days, discontinue use of this product and consult
a physician
Keep out of reach of children. If swallowed, get medical
help or contact a Poison Control Center right away.
| ASSURED
lidocaine hydrochloride gel |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Anhui NHU Pharmaceutical Co., Ltd. (530897792) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Anhui NHU Pharmaceutical Co., Ltd. | 530897792 | manufacture(69571-002) | |