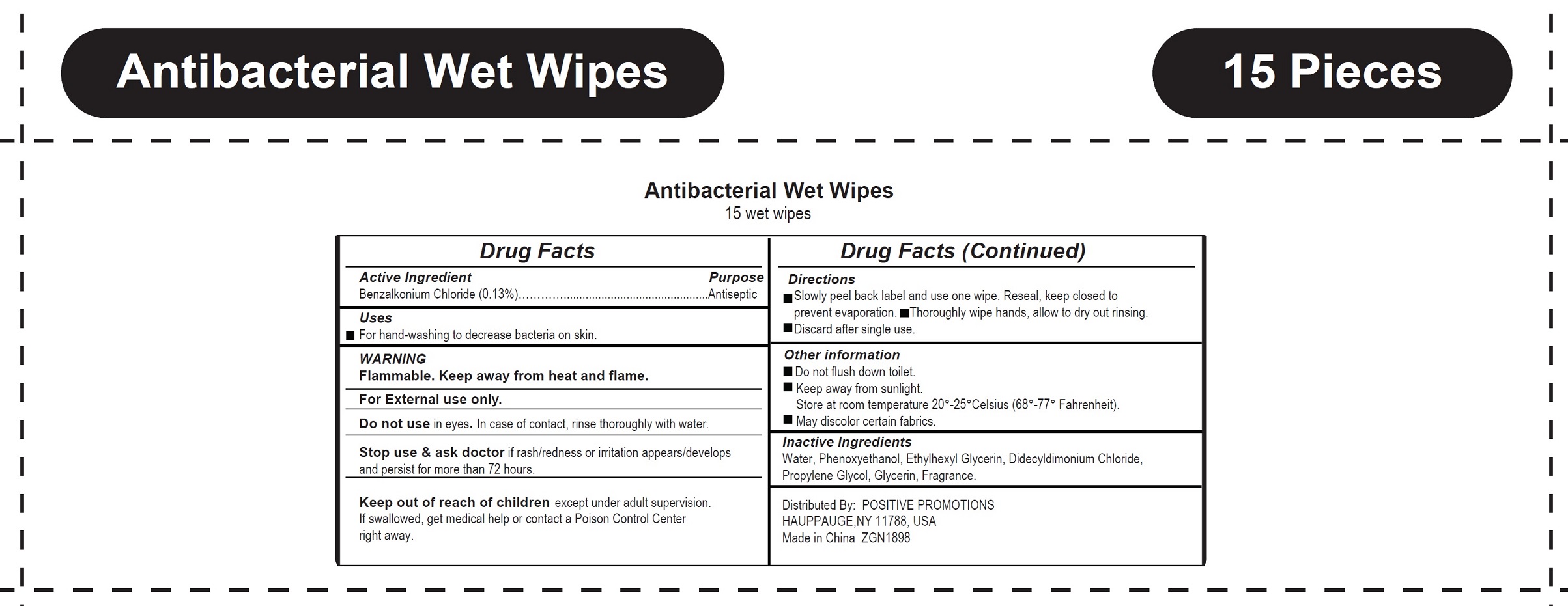
Antibacterial Wet Wipes by Positive Promotions Inc. Antibacterial Wet Wipes
Antibacterial Wet Wipes by
Drug Labeling and Warnings
Antibacterial Wet Wipes by is a Otc medication manufactured, distributed, or labeled by Positive Promotions Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTIBACTERIAL WET WIPES- benzalkonium chloride cloth
Positive Promotions Inc.
----------
Antibacterial Wet Wipes
WARNING
Flammable. Keep away from heat and flame.
For External use only.
Directions
- Slowly peel back label and use one wipe. Reseal, keep closed to prevent evaporation.
- Thoroughly wipe hands, allow to dry out rinsing.
- Discard after single use
Other information
- Do not flush down toilet.
- Keep away from sunlight. Store at room temperature 20°-25°Celsius (68°-77° Fahrenheit)
- May discolor certain fabrics
| ANTIBACTERIAL WET WIPES
benzalkonium chloride cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Positive Promotions Inc. (002401719) |
Revised: 1/2024
Document Id: 0fa1bbcd-97f9-f95b-e063-6394a90a5452
Set id: ba98494a-b23c-479e-b264-05d5c284430a
Version: 4
Effective Time: 20240123